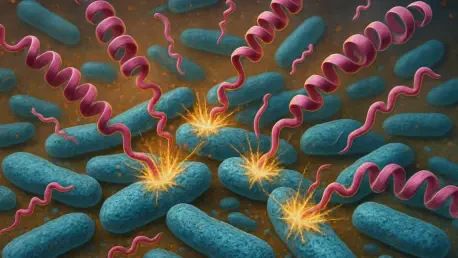
What if a microscopic weapon, hidden within the human body, could be the key to defeating one of the deadliest health threats of modern times—antibiotic-resistant bacteria, which claim millions of lives each year due to infections that no longer respond to traditional treatments? This urgent crisis
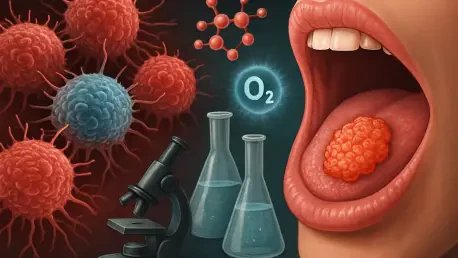
Imagine a battlefield within the human body where oral cancer, a devastating form of head and neck cancer impacting hundreds of thousands globally each year, relentlessly adapts to survive against modern medical interventions. Despite significant advancements in surgery, radiation, and
In the ever-evolving field of pharmaceutical development, the quest for safer and more effective drugs remains a critical challenge, especially when it comes to covalent inhibitors—a class of drugs known for their potent action but also their potential for harmful side effects. A groundbreaking

The battle against pediatric cancer remains one of the most emotionally charged and urgent challenges in modern healthcare, impacting thousands of young lives across the United States each year with diagnoses that shatter families and communities. Despite progress in medical science, many children

In the fast-evolving world of pharmaceuticals, the first half of this year has delivered a stunning reshuffle in the hierarchy of blockbuster drugs, with Novo Nordisk’s Ozempic and Eli Lilly’s Mounjaro vaulting into the top three revenue earners. This dramatic rise, fueled by an insatiable demand

In a groundbreaking development that bridges traditional healthcare research with cutting-edge blockchain technology, a Nasdaq-listed cancer research company has unveiled a staggering $344.4 million digital asset treasury, marking a significant pivot from its core mission of AI-driven drug