The intersection of severe influenza during pregnancy and its potential to harm fetal brain development has emerged as a critical concern for both expectant mothers and medical professionals, prompting urgent attention. A pioneering study conducted by researchers at the University of Illinois
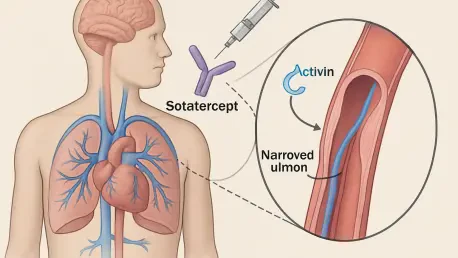
Pulmonary arterial hypertension (PAH), a life-threatening condition characterized by dangerously high blood pressure in the lung arteries, has long posed significant challenges for patients and clinicians alike due to its progressive nature and limited treatment options. Enter sotatercept, a

I'm thrilled to sit down with Ivan Kairatov, a renowned expert in environmental health and vision science, whose groundbreaking work has shed light on the critical link between air quality and children's eyesight. With a deep background in biopharma and a passion for leveraging technology to
Imagine a world where a single injection in the arm could halt the progression of a debilitating eye condition like age-related macular degeneration (AMD), a disease that affects nearly 200 million people globally and stands as a leading cause of vision loss among those over 60. This vision is
For countless families touched by Huntington’s disease (HD), a genetic neurodegenerative disorder with no cure or disease-modifying treatments, the struggle against an inevitable decline has been a heartbreaking reality, but a beacon of hope emerged on September 24, 2025. On that date, uniQure
In a remarkable achievement that underscores a steadfast dedication to excellence in pediatric medical imaging, Children’s Hospital of Orange County (CHOC), part of Rady Children’s Health, has been named an inaugural recipient of the BeRAD Professionalism Award by the American Society of Radiologic