A recent scientific breakthrough has shed light on a promising new strategy against ovarian cancer, demonstrating how a natural compound derived from a traditional herb can selectively destroy malignant cells by disrupting their internal waste management system. The study, a collaborative effort

The immune system's most sophisticated soldiers, B cells programmed for a single mission, are now understood to possess a startling ability to shed their identity and revert to a dangerously flexible state. Groundbreaking research has unveiled a surprising twist in cellular biology, demonstrating

For patients diagnosed with the notoriously aggressive small cell lung cancer, the initial success of treatment often conceals a brutal truth about the formidable battle that lies ahead. This particular form of lung cancer presents one of modern oncology's most persistent and heartbreaking
A profound paradox exists within modern pharmacology where the treatments designed to combat deadly diseases can simultaneously inflict severe and lasting damage on the human brain. For countless individuals undergoing treatment for conditions like HIV and cancer, this is a harsh reality; up to
Within the burgeoning field of regenerative medicine, the value of umbilical cord blood as a potent and ethically sound source of life-saving stem cells is universally recognized, offering transformative treatments for a spectrum of conditions from genetic disorders to hematologic cancers. The
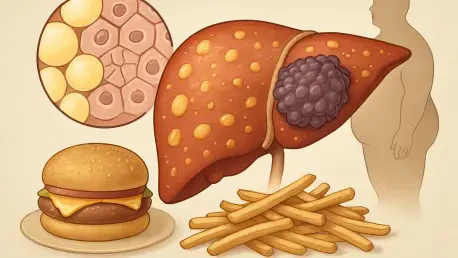
Beyond the familiar warnings about calories and cholesterol, a high-fat diet may be silently orchestrating a dangerous coup within the liver, transforming loyal, hardworking cells into precursors for malignant tumors. A landmark study from the Massachusetts Institute of Technology provides a