
The biopharmaceutical landscape is in a state of rapid evolution, driven by the increasing complexity of therapeutic modalities and the relentless pressure to accelerate timelines from discovery to market. In this high-stakes environment, the traditional, task-oriented relationship between drug

The foundational technologies that underpin modern medicine are undergoing a profound transformation, positioning the global cell culture consumables and equipment market for unprecedented expansion. Projections indicate that this critical sector, valued at US$ 13.06 billion in 2025, is on a

A multi-billion dollar investment in a single location can signal many things, but when a global healthcare leader repeatedly commits to the same state, it transforms from a business decision into a powerful statement about the future of American manufacturing. The recent groundbreaking for Johnson

The long-standing, methodical pace of clinical drug development may soon be disrupted by a statistical revolution endorsed at the highest regulatory levels, promising to make trials more flexible, efficient, and patient-focused. For decades, the path to drug approval has been paved with rigid,
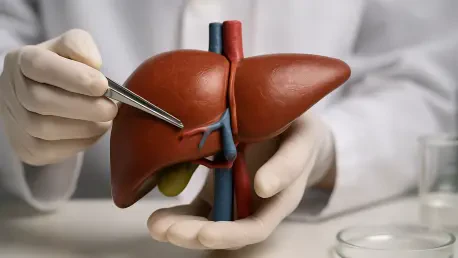
The human liver stands as a masterpiece of biological engineering, performing hundreds of vital functions that range from metabolic regulation and detoxification to the synthesis of essential proteins. This organ’s incredible functional complexity is matched by its unique mechanical profile, which
For decades, the intricate inner workings of biological tissues have remained largely hidden, with traditional microscopy methods only able to offer two-dimensional glimpses or superficial views of complex three-dimensional structures. This limitation has created a significant bottleneck in