
The microscopic world teeming within the human body harbors countless secrets, with recent discoveries now implicating a common fungus not as a mere passenger but as a sinister co-conspirator in the spread of deadly skin cancer. This revelation challenges conventional understanding by suggesting

The British monarchy, an institution historically defined by its stoic reserve, took an unprecedented step in 2024 when both King Charles and Catherine, the Princess of Wales, publicly disclosed their cancer diagnoses, shattering centuries of protocol that shrouded royal health in secrecy. This
A groundbreaking study led by scientists at The Wistar Institute marks a significant leap forward in the decades-long search for an effective HIV vaccine, demonstrating that a novel vaccine candidate can induce neutralizing antibodies after just a single immunization in nonhuman primates. This

With us today is a leading expert in biopharmaceutical innovation, here to break down a remarkable new development in diagnostic technology. We'll be exploring a novel biosensor test strip that promises to detect disease markers with unprecedented sensitivity, potentially moving complex diagnostics
The successful conclusion of cancer treatment should be a moment of pure triumph, yet for many, it signals the start of a lifelong struggle with chronic, painful tissue damage—a silent epidemic born from the very therapy that saved them. While radiotherapy stands as a pillar of modern oncology, its
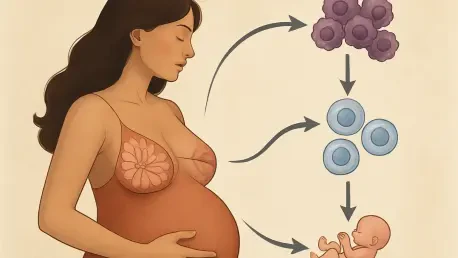
A long-standing paradox in oncology has puzzled researchers for decades: while increasing age is a primary risk factor for breast cancer, women who experience their first pregnancy before the age of 30 receive a remarkable and lifelong reduction in their risk. Recent research from cell biologists