
Imagine a world where a simple, noninvasive test could detect prostate cancer with pinpoint accuracy, sparing countless men from the anxiety and discomfort of unnecessary biopsies, and potentially transforming the landscape of early diagnosis. Prostate cancer remains a leading cause of

What happens when the joy of expecting a child is overshadowed by the shadow of life-threatening heart conditions? For countless women with cardiovascular disease, this is not a hypothetical question but a harsh reality that they face every day. Across the globe, maternal heart disease stands as

Imagine a world where a tiny camera, no larger than a grain of rice, can peer into the depths of coronary arteries to uncover hidden dangers that could lead to a devastating heart attack, a reality now being shaped by cutting-edge technology. Recurrent heart attacks strike approximately 15% of
In the ever-evolving landscape of cardiovascular care, staying ahead of diagnostic challenges is paramount for healthcare professionals striving to improve patient outcomes across diverse clinical settings. At the European Society of Cardiology (ESC) Congress held from August 29 to September 1 at
What if a tiny fraction of people held the key to eradicating one of humanity's most persistent viruses, offering hope to the over 38 million individuals living with HIV worldwide? This virus has defied a universal cure for decades, yet in a handful of remarkable cases, some have walked away

In a world where the journey from scientific discovery to life-saving treatment often spans decades, Emory University in Atlanta, Georgia, is taking bold steps to accelerate this process with the launch of its Center for New Medicines (CNM). Established just last year, this pioneering initiative
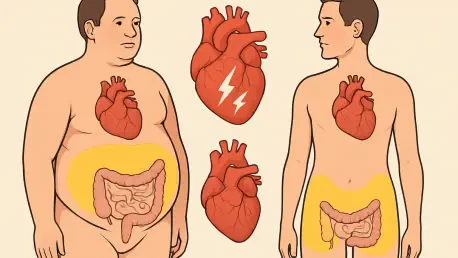
Imagine a world where a simple number like body mass index (BMI) no longer dictates how heart disease risks are assessed, and instead, the focus shifts to where fat resides in the body, marking a significant change in medical perspectives. This isn’t a distant dream but a pressing reality in 2025,
In a world where medical breakthroughs are increasingly vital to combat complex diseases, a remarkable discovery by researchers from the University of Utah and Sethera Therapeutics has captured the attention of the biotech community with a novel enzyme known as PapB. This advancement promises to
In the heart of the Midwest, where access to top-tier healthcare can sometimes be a challenge, Saint Luke's Health System emerges as a vital lifeline for communities across Missouri and Kansas, redefining exceptional medical care. Renowned for its extensive network of facilities and a steadfast

I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with a wealth of experience in research, development, and the integration of cutting-edge technology in healthcare. Today, we’re diving into a groundbreaking application of generative AI in public health—specifically, its role
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy