
In the heart of the Midwest, where access to top-tier healthcare can sometimes be a challenge, Saint Luke's Health System emerges as a vital lifeline for communities across Missouri and Kansas, redefining exceptional medical care. Renowned for its extensive network of facilities and a steadfast

I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with a wealth of experience in research, development, and the integration of cutting-edge technology in healthcare. Today, we’re diving into a groundbreaking application of generative AI in public health—specifically, its role

In an era where digital innovation is reshaping every facet of daily life, telemedicine has surged to the forefront of healthcare, fundamentally altering how primary care is accessed and delivered. Sparked by the urgent needs of the COVID-19 pandemic, this shift to virtual consultations has opened
What if a rare and deadly form of lung cancer, long considered a near-hopeless battle, suddenly had a new weapon in its fight? Picture a patient, grappling with the weight of a diagnosis of HER2-mutant non-small cell lung cancer (NSCLC), now finding a glimmer of possibility with an oral therapy

Imagine a scenario where a newborn in Qatar is screened for life-threatening genetic conditions before even leaving the hospital, with results so accurate that families can avoid years of uncertainty and distress. This is no longer a distant dream but a reality through the BeginNGS program, a

What if a technology could scan your lifestyle, preferences, and health needs to craft a perfect meal plan just for you? In a world where obesity rates have soared—with over 1.9 billion adults classified as overweight globally by the World Health Organization—the struggle to eat healthily feels

Imagine a political landscape where access to health care hinges on the drawing of district lines, a scenario unfolding right now as advocacy groups and lawmakers clash over congressional maps in a heated battle for influence. With reproductive rights and health funding under threat from
What happens when a relentless cancer, deemed incurable, meets a groundbreaking therapy that defies the odds? For the 15,000 Americans diagnosed annually with follicular lymphoma—a slow-growing but persistent form of non-Hodgkin lymphoma—this question is no longer hypothetical. A stunning phase 3
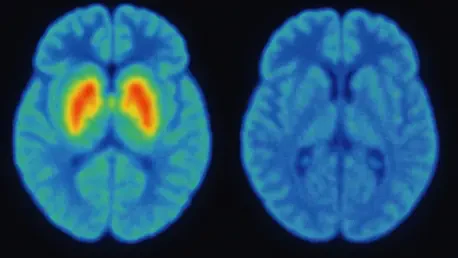
Imagine a world where a devastating condition like Parkinson’s disease (PD), which impacts millions globally with its debilitating tremors, stiffness, and slowed movements, can be detected long before symptoms disrupt daily life. This progressive neurodegenerative disorder stems from the loss of
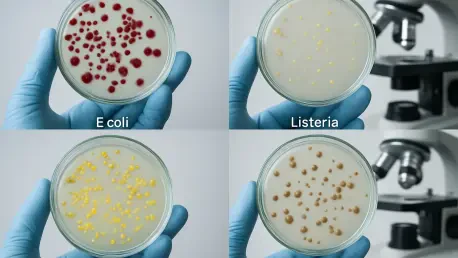
What happens when the very food meant to nourish us becomes a silent carrier of deadly threats? Each year, an estimated 600 million people worldwide fall ill from foodborne illnesses, with 420,000 losing their lives to pathogens that have grown resistant to traditional defenses, posing a growing
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy