Researchers at the Perelman School of Medicine at the University of Pennsylvania have made a significant breakthrough in personalized medicine and cancer treatment. By using patient-derived glioblastoma (GBM) organoids, they have developed a method to model patient responses to CAR-T cell therapy in real time. This innovative approach offers new insights into personalized treatment strategies for this aggressive form of brain cancer.
Real-Time Modeling of Patient Responses
Leveraging Lab-Grown Organoids
The study began with patients who had recurrent GBM and were part of a Phase I clinical trial for a dual-target CAR-T cell therapy. Researchers created tumor organoids from the patients’ biopsied tumors and treated them in the same timeframe as the patients received CAR-T cell therapy. The primary goal was to determine if the organoids’ reactions to the therapy mirrored the actual tumors’ reactions in the patients’ brains. The findings indicated that if an organoid responded to treatment by shrinking, the patient’s tumor followed the same course.
The innovative use of organoids in real-time clinical settings addresses significant challenges in treating GBM. Due to the inability to frequently biopsy a patient’s brain and the difficulty in distinguishing between tumor growth and treatment-related inflammation through MRI, accurate assessment of patient response to treatment has been challenging. Organoids, which reflect the patient’s brain environment with striking accuracy, can help tailor personalized therapies more effectively.
Overcoming Treatment Hurdles
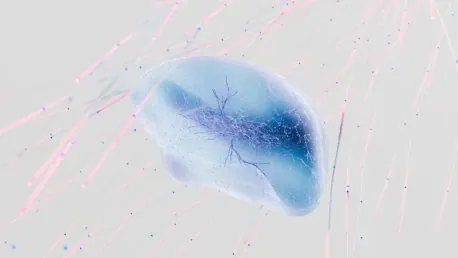
The creation of GBM organoids marks a breakthrough in replicating the tumor’s diverse cellular environment, which includes various cancer cell types, immune cells, blood vessels, and other tissue. This complexity has historically made GBM hard to treat since conventional models couldn’t mimic the tumor’s environment accurately. However, the researchers’ method of growing organoids using small pieces of a patient’s actual tumor—not just a single type of cancer cell—overcomes this limitation, offering a more robust and accurate model for therapeutic testing.
CAR-T therapy works by reprogramming a patient’s T cells to identify and destroy specific cancer cells. Although this technique has seen success in treating blood cancers, it has faced challenges with solid tumors like GBM due to the tumors’ heterogeneity and complex, immunosuppressive microenvironments. Recent studies suggest targeting two brain tumor-associated proteins might be an effective strategy for reducing tumor size in patients with recurrent GBM.
Creating Accurate Tumor Models
Duplicating the Tumor’s Diverse Environment
Researchers noted that while organoids have been employed in retrospective studies, they haven’t been predominantly used in real-time clinical settings. This novel approach, integrating organoids with patient-matched treatments, is groundbreaking. It enhances the potential of organoids to test treatment responses promptly and could aid in determining the most efficacious personalized treatment options for individual patients.
GBM is known for being aggressive and is the most common cancerous brain tumor in adults, with a grim life expectancy of just 12-18 months post-diagnosis. Currently available treatments—surgery, radiation, and chemotherapy—unfortunately offer limited success in extending patients’ lives. The research team created organoids from six patients with recurrent GBM participating in the clinical trial, and as creating sufficient cancer cells in the lab for testing typically takes months, organoid generation in this study took merely 2-3 weeks while patients recuperated from surgery and before starting CAR-T therapy.
CAR-T Therapy and Solid Tumors
Significant in the findings, the response of the organoids to CAR-T cell treatment was consistent with how the actual tumors responded in the patients’ brains. When an organoid underwent cancer cell destruction by T cells, the patient’s MRI scans also showed reduced tumor size, accompanied by an increased presence of CAR-positive T cells in their cerebrospinal fluid, affirming the efficacy of the treatment.
Neurotoxicity is a common concern with CAR-T cell therapy, where toxic effects alter the nervous system’s function, potentially killing brain cells. The study observed similar levels of immune cytokines (indicators of toxicity) in both the organoids and the patients’ cerebrospinal fluids. These levels decreased significantly a week post-treatment, suggesting that organoids can also accurately model a patient’s risk of neurotoxicity, helping clinicians to determine suitable CAR-T cell dosages.
Real-Time Clinical Applications
Integrating Organoids with Patient-Matched Treatments
The study demonstrated a high correlation between various parameters, including the degree of organoid cytolysis in vitro and the actual CAR-T cell engraftment in patients. The time course of cytokine release in organoids correlated well with patient cerebrospinal fluid levels, offering additional critical insights into patient treatment responses.
Co-senior study authors, including Hongjun Song, PhD, and Guo-li Ming, MD, PhD, highlighted the potential of these organoids to understand patient-specific tumor behavior and treatment responses accurately. They envisaged this breakthrough leading to more personalized treatment regimens and advancing the understanding of GBM’s complexities.
Addressing GBM’s Aggressiveness
Researchers at the Perelman School of Medicine at the University of Pennsylvania have achieved a pivotal advancement in the fields of personalized medicine and cancer treatment. They have utilized patient-derived glioblastoma (GBM) organoids to develop a methodology for modeling patient responses to CAR-T cell therapy in real time. This cutting-edge technique presents a novel way to gain insights into individualized treatment strategies for this highly aggressive type of brain cancer. Glioblastoma is notoriously difficult to treat due to its complex and rapidly changing nature, making the discovery exceptionally crucial. By creating organoids, which are essentially miniaturized versions of the patient’s tumor, scientists are able to observe how specialized CAR-T cells interact with the cancer in a laboratory setting. This allows for more accurate predictions about how a patient will react to specific therapies, enabling doctors to tailor treatments to each individual’s unique cancer profile. Consequently, this approach is set to revolutionize the treatment paradigm, providing hope for more effective and personalized cancer care in the future.