
Managing severe, persistent respiratory conditions often feels like a relentless battle, with frequent treatments and the constant threat of debilitating flare-ups dictating the rhythm of daily life. For millions, conditions like severe asthma and chronic rhinosinusitis with nasal polyps represent
A groundbreaking Phase III clinical trial has revealed that the therapy Gazyva (obinutuzumab) demonstrates superior efficacy over the current standard-of-care treatment, tacrolimus, for adults grappling with primary membranous nephropathy. The MAJESTY trial’s success represents a significant

A single rogue protein, present in nearly every person diagnosed with amyotrophic lateral sclerosis, has long been the focus of intense scientific pursuit and a major roadblock to effective treatment. Netherlands-based biotechnology company VectorY Therapeutics has now initiated a landmark clinical

The emergence of cell-cultured protein has sparked intense debate at the intersection of agricultural tradition and technological innovation, nowhere more so than in states with deep roots in the livestock industry. In South Dakota, a legislative effort to completely prohibit the sale of lab-grown

In a move that sends ripples across the global pharmaceutical landscape, AstraZeneca has committed to a landmark $15 billion investment in China, a strategic initiative designed to run through 2030. The announcement, made on January 29, 2026, signals a profound deepening of the company's research,
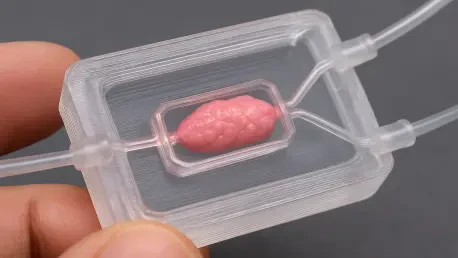
Researchers have developed a groundbreaking microfluidic chip using a single-step 3D printing process that successfully mimics the three-dimensional environments where cells grow in the human body, a development poised to revolutionize how diseases are studied and new drugs are tested. This new