
In an era where technology is reshaping every facet of society, the healthcare sector stands at a critical juncture, grappling with the integration of artificial intelligence (AI) to revolutionize governance and uphold stringent standards. The rapid adoption of digital tools and AI systems is not

In an age where artificial intelligence is reshaping the healthcare landscape with unprecedented speed, the protection of sensitive patient data has emerged as a paramount concern for industry leaders and policymakers alike, especially as cybercriminals increasingly target healthcare systems. Drawn

A startling revelation has emerged from Arizona’s Medicaid program, known as the Arizona Health Care Cost Containment System (AHCCCS), raising serious questions about oversight and accountability at the highest levels. The chief medical officer, a pivotal figure in shaping health policies for some
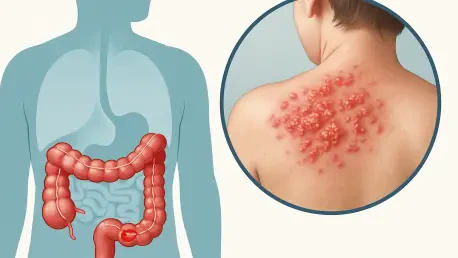
In a world where health challenges often intersect, understanding the relationship between conditions like colon cancer and shingles becomes crucial for effective management and prevention, especially since both can significantly impact quality of life when they occur simultaneously. This creates a
In a world where the immune system stands as both protector and potential adversary, the announcement of this year’s Nobel Prize in Physiology or Medicine has captured global attention with its profound implications for human health. Awarded to Mary E. Brunkow, Fred Ramsdell, and Shimon Sakaguchi,
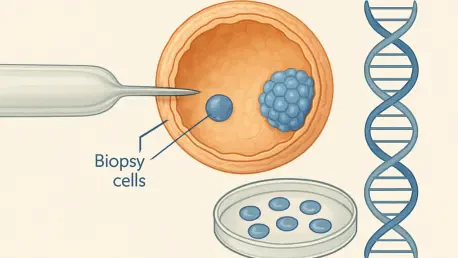
Recurrent pregnancy loss, often referred to as RPL and characterized by two or more consecutive miscarriages, casts a heavy shadow over the dreams of countless couples hoping to build a family. For those navigating the complex world of in vitro fertilization (IVF), a beacon of hope emerges through