
What happens when a beacon of hope for millions battling multiple sclerosis (MS) dims in a single clinical trial? In a field where breakthroughs are rare and desperately needed, Contineum Therapeutics, a San Diego-based biotech firm, has hit a significant roadblock with their lead drug, PIPE-307,

Diving into the world of virology, we’re thrilled to speak with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development. With a deep understanding of technology and innovation in the industry, Ivan has been at the forefront of groundbreaking discoveries.
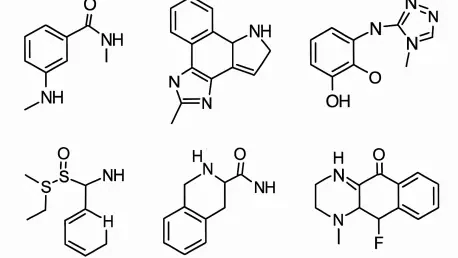
In the intricate landscape of drug discovery, pinpointing effective inhibitors for Protein Tyrosine Phosphatase 1B (PTP1B) stands as a monumental challenge with profound implications for treating metabolic disorders such as diabetes and obesity. PTP1B is a key player in regulating insulin signaling

In a landscape where lung cancer remains one of the most challenging diseases to treat, particularly for rare subsets of patients, a new development has sparked hope among clinicians and affected individuals alike. Bayer’s recently approved drug, sevabertinib, marketed as Hyrnuo, has gained

In the intricate world of drug development, where every trial holds the potential to transform lives, a quiet revolution is unfolding in the unassuming spaces of suburban medical corridors, reshaping how research reaches communities. Outpatient clinics, traditionally viewed as mere venues for
What happens when the building blocks of life face destruction before they can fulfill their purpose, and how do cells protect these vital components during such a delicate process? Inside every cell, a precise mechanism unfolds where proteins, essential for everything from immunity to growth, are