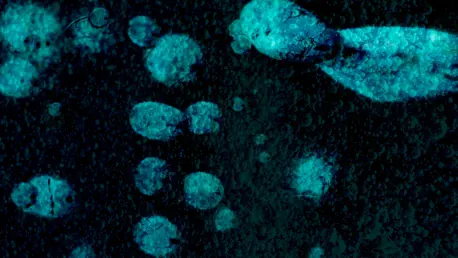
Cryptococcus neoformans infections often pose a significant threat to individuals with weakened immune systems, such as those with HIV/AIDS or organ transplant recipients. These infections can lead to cryptococcosis, a severe condition that initially affects the lungs and may spread to the brain,

The effectiveness of cannabidiol (CBD) in reducing anxiety among patients with advanced breast cancer has become an intriguing subject for researchers, especially in the context of scan-related anxiety. A recent study conducted by researchers from the Dana-Farber Cancer Institute and Mass General
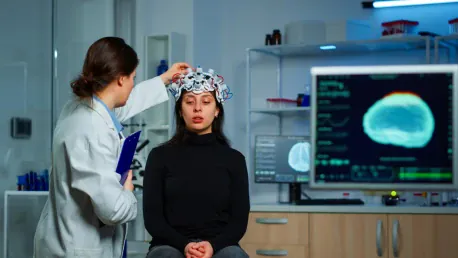
Rett syndrome is a severe neurological disorder primarily affecting young girls, leading to significant cognitive, motor, and communication impairments. The disorder is linked to mutations in the MeCP2 gene, which encodes the methyl-CpG binding protein 2 (MeCP2). Researchers have long sought to

A drug initially celebrated for its potential in treating a rare liver disease has come under scrutiny following alarming safety findings. Ocaliva, developed by Intercept Pharmaceuticals and now under Alfasigma's ownership, was initially viewed as a breakthrough treatment for primary biliary

The recent endorsement of Neuraxpharm's ublituximab (BRIUMVI®) by the National Institute for Health and Care Excellence (NICE) marks a significant step forward in the treatment of relapsing-remitting multiple sclerosis (RRMS). This approval is a notable accomplishment for the European specialty
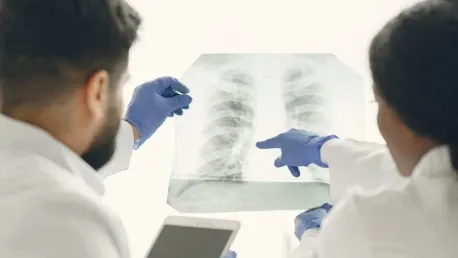
The recent endorsement by the National Institute for Health and Care Excellence (NICE) to utilize AstraZeneca's Imfinzi (durvalumab) in combination with chemotherapy as a treatment for adults with resectable non-small cell lung cancer (NSCLC) marks a significant milestone in cancer care. This