
In the rapidly evolving landscape of life sciences, the challenges plaguing drug development have become more pronounced, prompting an urgent need for transformation. The industry has long grappled with prolonged cycle times, substantial costs, and steep complexities in clinical trials that delay

The recent inauguration of the NIHR Bradford and West Yorkshire Commercial Research Delivery Centre (CRDC) marks a significant milestone in enhancing healthcare access in West Yorkshire. Established by the Bradford Teaching Hospitals NHS Foundation Trust, this clinical research hub is part of an
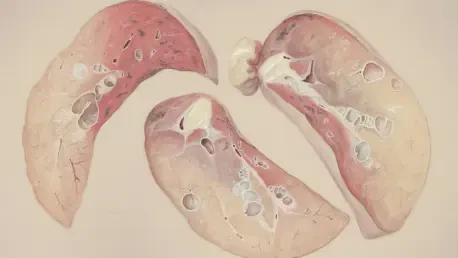
In recent months, the world of oncology has witnessed a remarkable milestone, bringing a glimmer of hope to thousands of patients battling advanced lung cancer. With the US Food and Drug Administration (FDA) speeding up approval for a groundbreaking drug, the focus has intensified on a specific

Biovica International, known for its innovations in blood-based cancer monitoring, recently unveiled a strategic collaboration with a prominent US-based Tier-1 pharmaceutical company. This partnership reflects a mutual commitment to pioneering advancements in cancer treatment. The alliance
Recent developments have propelled the field of oncology drug development into a new era, primarily due to the integration of artificial intelligence. Startups like Pathos AI Inc. exemplify this shift by leveraging AI to streamline vital processes within oncology research. The company has

In the evolving landscape of clinical research, artificial intelligence (AI) has become an indispensable tool, yet the level of adoption and integration at clinical research sites remains inconsistent. AI's potential to enhance various aspects of clinical trials, from design to data management, has