In an era where medical advancements are reshaping how humanity approaches health, a transformative idea is gaining traction among scientists who argue that the key to tackling chronic diseases lies not in treating them individually, but in targeting the very biology of aging itself. This

What happens when the joy of motherhood is eclipsed by an unyielding fog of despair for nearly half a million women in the U.S. each year? Postpartum depression (PPD), a debilitating condition affecting about 15% of new mothers, often turns a time of bonding into a battle for mental survival. Amid
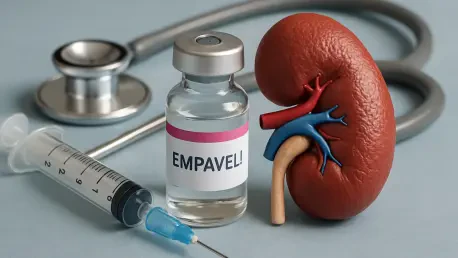
Imagine a world where thousands of Americans, including children, face the grim reality of kidney failure within a decade due to rare, debilitating conditions like C3 glomerulopathy (C3G) and primary immune complex membranoproliferative glomerulonephritis (IC-MPGN). These diseases, affecting around
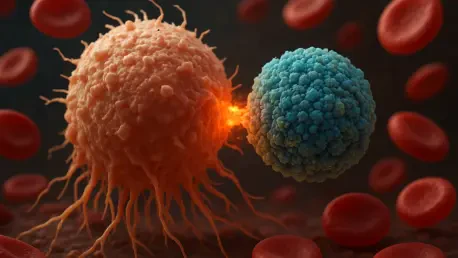
What happens when a rare cancer keeps coming back, defying every treatment thrown at it? For adults battling relapsed marginal zone lymphoma (MZL), a subtype of non-Hodgkin lymphoma, this relentless cycle has long been a harsh reality, but a flicker of hope emerges as Bristol Myers Squibb’s

In a groundbreaking development for medical science in Southern California, the University of California San Diego (UC San Diego) has been awarded an $80 million, seven-year grant from the National Center for Advancing Translational Sciences (NCATS), a division of the National Institutes of Health
Welcome to an insightful conversation with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, as well as a deep understanding of technological innovation in the industry. Today, we dive into the promising realm of GLP-1 receptor agonists (GLP-1 RAs)