
As the biotechnology sector continues to drive innovation in healthcare, September stands out as a critical month for several companies on the cusp of releasing pivotal clinical trial data that could reshape their futures. These upcoming announcements might serve as major catalysts, potentially
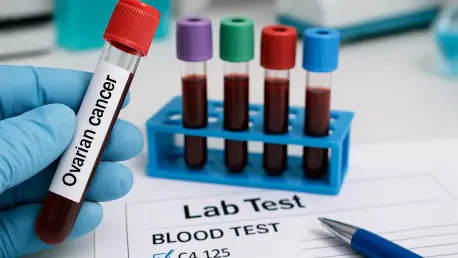
Imagine a world where a silent, deadly disease like ovarian cancer, which affects thousands of women each year, could be caught in its earliest stages with just a simple blood draw, offering hope for timely intervention. This devastating illness, often striking women over 50, frequently goes

I'm thrilled to sit down with Ivan Kairatov, a biopharma expert with extensive experience in research and development, and a deep understanding of technological innovation in the industry. Today, we’re diving into the groundbreaking work on novel monoclonal antibodies for mpox, a viral disease that

In a remarkable leap forward for obesity treatment, Eli Lilly has revealed significant strides with orforglipron, an innovative oral GLP-1 receptor agonist targeting both obesity and diabetes. The announcement on August 26 showcases results from a pivotal Phase 3 clinical trial that have ignited

Welcome to an insightful conversation with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, as well as a deep understanding of technological innovation in the industry. Today, we dive into the groundbreaking advancements in RNA-based therapies,

Imagine a condition so rare and devastating that it not only compromises the body’s ability to repair DNA but also skyrockets the risk of cancer by 500 to 700 times compared to the general population, a reality for individuals with Fanconi anemia (FA). This genetic disorder often leads to bone