
For millions of people who rely on proton pump inhibitors (PPIs) to manage chronic acid reflux and other gastrointestinal conditions, a persistent concern has long cast a shadow over their treatment: the potential risk of developing stomach cancer. This decades-old question has fueled anxiety among

The arduous journey of a new drug from a laboratory concept to a patient's hands is a monumental undertaking, an odyssey fraught with staggering costs, extensive research, and an alarmingly high probability of failure. For every ten promising drug candidates that enter the rigorous phase of human
The escalating crisis of metabolic disease is silently fueling a surge in liver cancer cases worldwide, forcing researchers to seek innovative strategies that can halt this deadly progression before it begins. A class of drugs originally developed for diabetes, known as nutrient-stimulated

For the first time in over half a century, the fundamental list of nutrients considered indispensable for human life may be on the verge of a historic update, with the candidate being a familiar yet profoundly misunderstood dietary component. A concerted effort by leading nutrition experts is
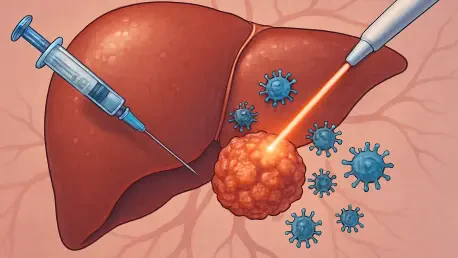
A groundbreaking therapeutic strategy that combines a systemic targeted drug with a standard locoregional treatment is showing unprecedented promise in preventing the recurrence of hepatocellular carcinoma (HCC) among high-risk patients who have undergone surgery. This comprehensive analysis of a

Today we're speaking with Ivan Kairatov, a biopharma expert with deep insights into the technological innovations shaping modern medicine. We'll be delving into a remarkable breakthrough in oncology: a new, highly targeted procedure for treating metastatic uveal melanoma, a rare and aggressive eye