
The intricate landscape of the tumor microenvironment is increasingly recognized not as a simple collection of malignant cells, but as a complex ecosystem where cancer’s fate is dictated by a dynamic interplay with the body's own immune system. The targeting of specific immune cell subsets

A significant European collaborative initiative has made remarkable strides in understanding the intricate dance between the body's immune system and the onset of cancer, potentially unlocking a new era of predictive diagnostics. By focusing on the often-overlooked first-line defenders known as
A baffling contradiction has long stood at the heart of understanding how one of the most aggressive forms of breast cancer spreads throughout the body, but new research has finally unraveled this mystery, revealing a unique survival strategy that cancer cells use to become metastatic. For years,
The promise of harnessing the body's own immune system to fight cancer has been one of the most significant medical breakthroughs of the century, yet a persistent paradox has puzzled clinicians and researchers alike. For years, the prevailing wisdom held that tumors with more genetic mutations were

Within the intricate machinery of our cells, the iconic double helix of DNA has long been considered the fundamental blueprint of life, but recent breakthroughs have revealed that our genetic code can adopt far more complex and enigmatic shapes. A comprehensive study has illuminated the existence
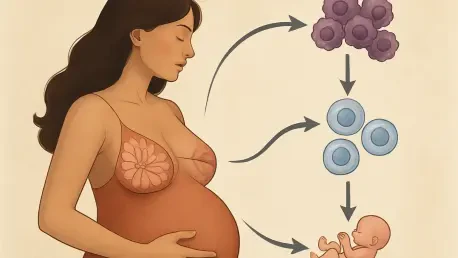
A long-standing paradox in oncology has puzzled researchers for decades: while increasing age is a primary risk factor for breast cancer, women who experience their first pregnancy before the age of 30 receive a remarkable and lifelong reduction in their risk. Recent research from cell biologists