
The European health sector is currently navigating a period of unprecedented transformation, where landmark legislative reforms and a surge in therapeutic innovation are converging to redefine the future of medicine regulation. As the European Medicines Agency (EMA) marks its 30th anniversary, it
Long before a name is forgotten or a memory fades, a silent war is waged within the brain, and a new pharmaceutical weapon may have just arrived to fight it on its own terms. This battleground, previously invisible, is now the focus of groundbreaking research from Northwestern University, where

The longstanding quest to measure the true pace of human aging, independent of the calendar, has taken a decisive turn with the development of a biomarker that can be read from a simple urine sample. The development of the urinary microRNA (uEV-miRNA) aging clock represents a significant
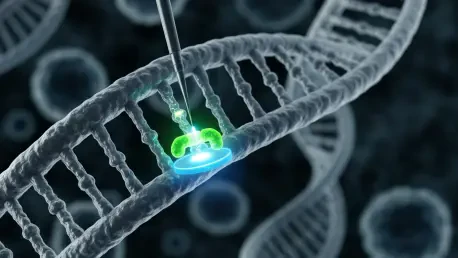
The intricate dance of cellular machinery required to produce life-saving biologic therapies has long been dictated by a rhythm of chance, but a new era of genomic precision is fundamentally changing the tempo of drug development. For decades, the biopharmaceutical industry has grappled with a

In the high-stakes world of biopharmaceutical manufacturing, where a single production run can be valued in the millions of dollars, the quiet disposal of a failed batch represents a catastrophic loss of resources, time, and patient trust. While the industry strives for perfection, the reality is

The body's intricate system for manufacturing blood cells is a marvel of biological engineering, but when this internal factory fails, the consequences are catastrophic, leaving patients defenseless against infection and uncontrolled bleeding. For individuals diagnosed with severe aplastic anemia