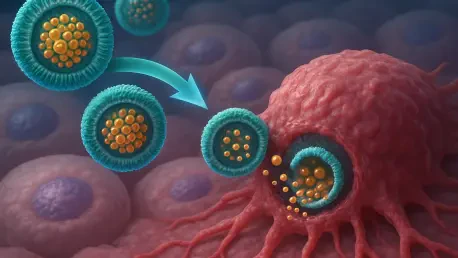
I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, particularly in cutting-edge technologies and innovations in the industry. Today, we’re diving into a groundbreaking advancement in cancer treatment—a new formulation of
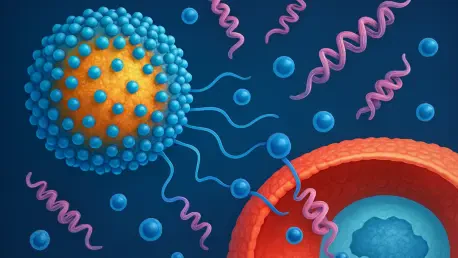
Imagine a world where life-saving treatments for cancer, autoimmune diseases, and metabolic disorders no longer require hours of intravenous infusions in a clinical setting, allowing patients to administer their medications at home with a quick injection, reclaiming time and autonomy. This scenario
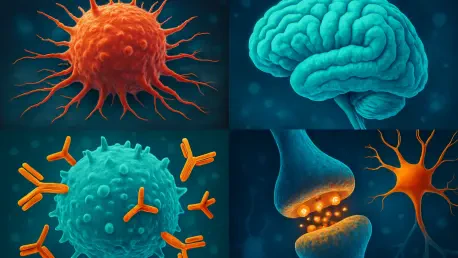
In a remarkable stride forward for cancer treatment, researchers have uncovered a profound connection between cancer cells, the nervous system, and immune responses, shedding light on why some patients resist immunotherapy. This emerging field, known as cancer neuroscience, explores how tumors
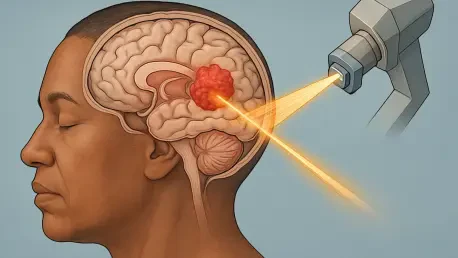
Imagine a diagnosis so devastating that it offers a median survival of just one year, primarily striking children and young adults with little hope for effective intervention. Diffuse midline glioma with an ### K27M mutation, an ultra-rare and aggressive brain cancer, affects roughly 2,000
What happens when a relentless cancer, deemed incurable, meets a groundbreaking therapy that defies the odds? For the 15,000 Americans diagnosed annually with follicular lymphoma—a slow-growing but persistent form of non-Hodgkin lymphoma—this question is no longer hypothetical. A stunning phase 3

In the intricate world of biopharmaceuticals, where the journey from drug discovery to patient delivery is fraught with challenges, the roles of Clinical Operations (ClinOps) and Medical Affairs (MA) stand out as indispensable. ClinOps serves as the operational backbone, meticulously managing