
In the United Kingdom, a pressing healthcare crisis has come to light, revealing that only about half of cancer patients receive a diagnosis within the targeted timeframe set by the National Health Service (NHS) in England, according to a recent study. Conducted by Cancer Research UK in
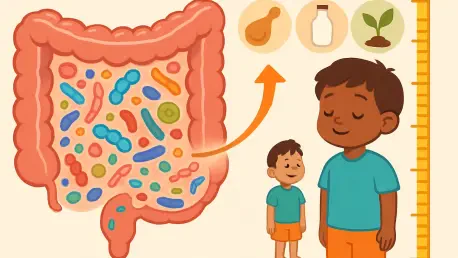
Imagine a world where millions of children struggle to grow, not just because of a lack of food, but due to invisible forces within their own bodies, affecting their development profoundly. Over 150 million children globally face undernutrition, a condition that stunts growth, impairs cognition,
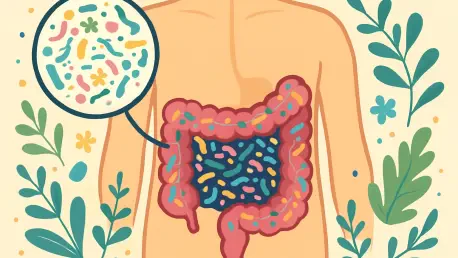
I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, particularly in the intersection of technology and innovation in health sciences. Today, we’re diving into the fascinating world of the gut microbiome, exploring

Imagine a world where a simple blood test could reveal hidden risks to your health long before symptoms appear, allowing for preventive measures that could save lives, and this is no longer a distant dream but a reality through a groundbreaking program offered by Parkview Health. Known as DNA

In a world where complex diseases like cancer and neurodegenerative disorders continue to challenge medical science, a transformative approach is emerging through artificial intelligence (AI). Imagine a tool so precise that it can predict the exact genetic targets needed to reverse a diseased
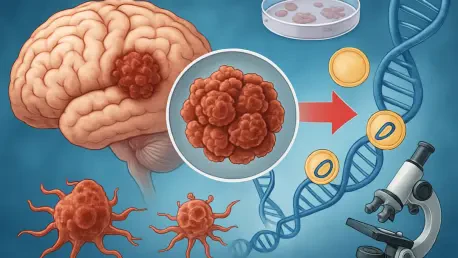
I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, particularly in cutting-edge technology and innovation within the industry. Today, we’re diving into a groundbreaking discovery about extrachromosomal DNA, or ecDNA, and