
In the realm of clinical research, the conclusion of a trial often feels like crossing a finish line, with key milestones such as database lock, the final monitoring visit, or the last patient out signaling closure for sponsors and contract research organizations (CROs). However, for the
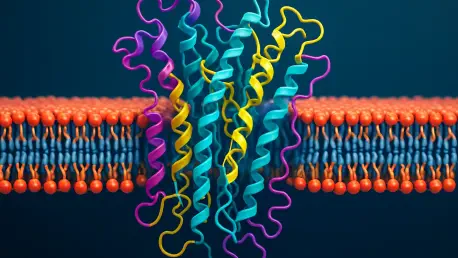
What if the key to defeating some of humanity's most devastating diseases lies within the tiniest boundaries of human cells, invisible to the naked eye, and only accessible through advanced technology? Membrane proteins, the unsung heroes of cellular function, act as gatekeepers, controlling the

I'm thrilled to sit down with Ivan Kairatov, a distinguished expert in biopharma with a profound understanding of technological innovation and research in the field. Today, we’re diving into the groundbreaking advancements in biomedical imaging, specifically focusing on a pioneering project at the

In an era where personalized medicine is becoming the cornerstone of healthcare, a revolutionary advancement in genetic testing is capturing attention for its potential to transform lives, particularly for those with mixed ancestry. Imagine a scenario where a patient, unaware of the intricate blend

In the intricate world of healthcare, patient safety culture stands as a cornerstone, embodying the shared values, attitudes, and behaviors that aim to minimize harm to patients during medical care, especially in high-stakes environments like operating theaters. In these settings, where complex
In a remarkable leap forward for medical science, researchers at McMaster University in Canada have introduced a groundbreaking antibiotic named enterololin , tailored specifically to combat inflammatory bowel disease (IBD), which encompasses debilitating conditions like Crohn's disease. This