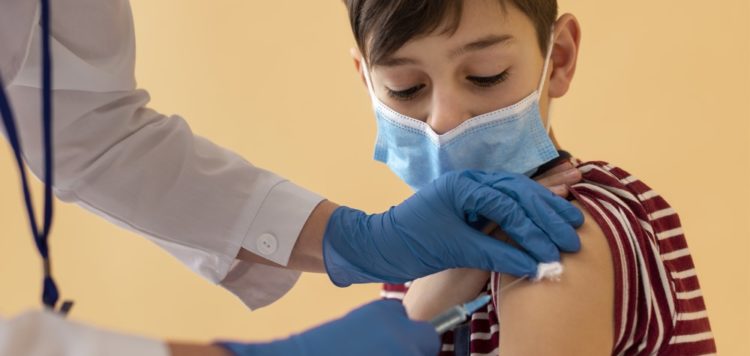
With coronavirus cases on the rise among children, Pfizer and BioNTech brought welcome news on Monday, reporting their COVID-19 vaccine is safe and generated a strong antibody response in kids aged 5 to 11.
The companies are the first COVID-19 vaccine makers to release data for children of this age. They will apply for emergency use authorization in the United States, Europe and elsewhere “as soon as possible,” the companies said.
In the phase 2/3 trial, 2,268 children received two 10 µg doses three weeks apart. Investigators observed antibody responses comparable to those seen in people aged 16 to 25 who received 30 µg doses, the companies said.