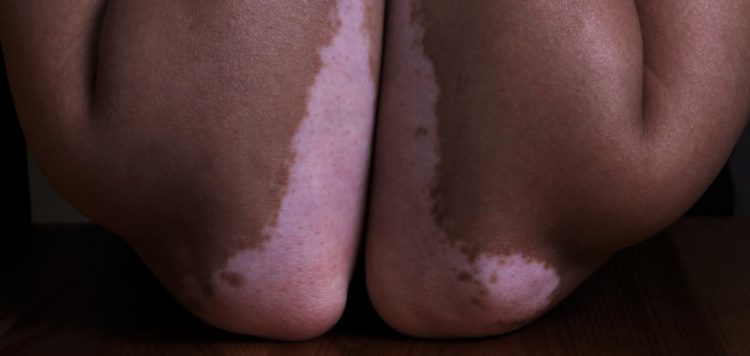
The FDA has kicked off a priority review of Opzelura (ruxolitinib), a JAK1/JAK2 inhibitor it approved to treat atopic dermatitis in September, and aims to decide on the vitiligo indication by 18 April next year.
If approved, Opzelura would provide an alternative to steroid drugs currently used to treat vitiligo, a chronic autoimmune disease that causes depigmentation of skin and affects around 1.5 million people in the US.
Results in vitiligo came from the TRuE-V1 and TRuE-V2 studies, which showed that around 30% of patients treated with ruxolitinib cream twice daily achieved 75% or more improvement from baseline in the facial vitiligo area scoring index at 24 weeks, significantly more than in a control group.