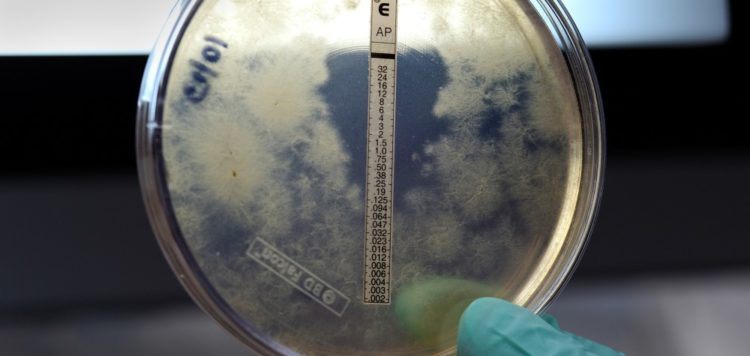
Rezzayo is part of a class of medications known as echinocandins, which are usually the first drugs used to treat patients suffering from bloodstream infections caused by Candida. Unlike the three approved medicines in the class, Rezzayo doesn’t require a daily injection.
The FDA approved Rezzayo for patients who have limited or no alternative treatment options based on Cidara research that the drug’s effects measured up to an existing medicine known as caspofungin. The new option has the potential to simplify treatment for patients, according to a statement from the principal researcher in Cidara’s pivotal trial, George Thompson of the University of California, Davis.