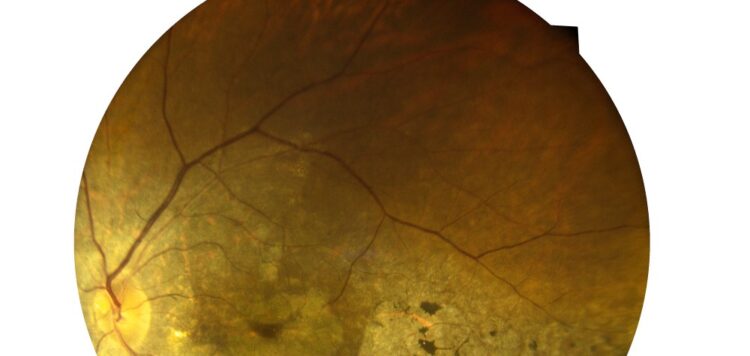
The U.S. Food and Drug Administration has approved Izervay (avacincaptad pegol intravitreal solution) for the treatment of geographic atrophy secondary to age-related macular degeneration.
The approval was based on two phase 3 clinical trials, in which patients received 2 mg intravitreal administration of Izervay. During a 12-month follow-up period, there was a statistically significant reduction in the rate of geographic atrophy growth in patients treated with Izervay versus sham. Slowing of disease progression was detected as early as six months, with up to a 35 percent reduction in the first year of treatment.