
Long before a name is forgotten or a memory fades, a silent war is waged within the brain, and a new pharmaceutical weapon may have just arrived to fight it on its own terms. This battleground, previously invisible, is now the focus of groundbreaking research from Northwestern University, where
A comprehensive analysis of a recent study has unveiled the pivotal role of a partner protein in supporting a key appetite-regulating protein, providing profound new insights into the molecular mechanisms governing human energy balance. This discovery, led by an international research team from the

By 2030, the tissue-engineering market is projected to exceed $32.45 billion , reflecting a profound shift in how biopharma is redefining the future of medicine. Once limited to academic labs, it has become a powerful engine of innovation in biopharma, transforming how businesses develop drugs,
Today we are joined by Ivan Kairatov, a biopharma expert with deep insights into the intersection of technology and medical innovation. For decades, the goal of HIV treatment has been management—using antiretroviral therapy to suppress the virus to undetectable levels. A true cure has remained

A groundbreaking analysis by Australian scientists has finally begun to unravel the complex biological puzzle of long COVID, identifying the key genetic and molecular drivers that cause debilitating symptoms to persist long after the initial infection has cleared. This pivotal research provides a
A landmark investigation into the intestinal microbiome has uncovered a remarkably specific and nuanced connection to the progression of multiple sclerosis (MS), revolving around a deceptive cellular mechanism known as "molecular mimicry." Collaborative research from the University of Basel and the

The intricate challenge of moving living, three-dimensional biological structures across countries and continents without compromising their integrity has long been a significant barrier to advancing global biomedical research. The development of non-cryogenic transport solutions represents a
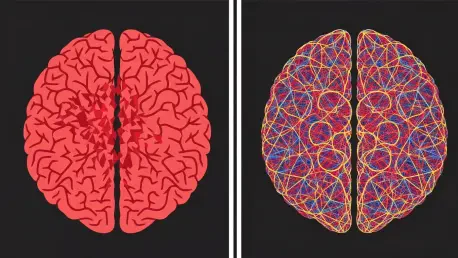
Today we're joined by Ivan Kairatov, a biopharma expert whose work at the intersection of neuroscience and artificial intelligence is paving the way for revolutionary new diagnostic tools. We'll be discussing a groundbreaking deep learning model that uses affordable, non-invasive EEG technology to

A transformative shift is underway in the treatment of Acute Myeloid Leukemia, offering renewed hope for patients battling the most aggressive and resistant forms of this devastating blood cancer. For years, the prognosis for individuals with relapsed or refractory AML has been bleak, with standard

Breast cancer remains one of the most significant health challenges worldwide, where the critical difference between a positive prognosis and a devastating outcome often hinges on the speed and precision of the initial diagnosis. For decades, the medical community has relied on established
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy