
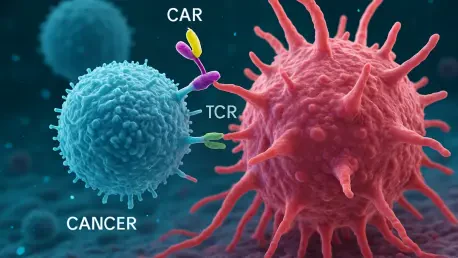
In the ceaseless battle against cancer, a remarkable stride forward has surfaced, promising to reshape the landscape of treatment options for patients worldwide. Chimeric Antigen Receptor T-cell (CAR-T) therapy, a cutting-edge immunotherapy that reengineers a patient’s immune cells to seek out and
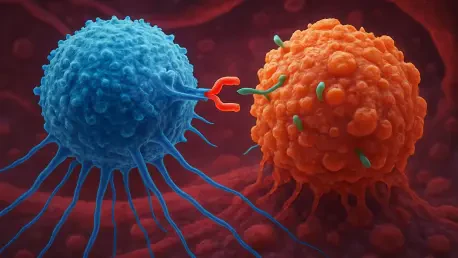
What if a cancer diagnosis no longer meant waiting weeks or months for a life-saving treatment, but instead, a solution could be engineered directly within the body in mere days? This isn’t science fiction—it’s the promise of in vivo CAR T cell therapy, a cutting-edge approach that could redefine
England faces an unprecedented public health challenge as projections indicate a staggering 6.3 million new cancer diagnoses by 2040, effectively doubling the current rate and resulting in a new case every two minutes, driven by an aging population and lifestyle factors such as smoking and obesity.
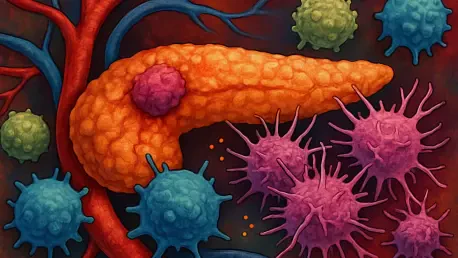
What if a single puff of cigarette smoke could turn the body's own defenses into a weapon for one of the deadliest cancers? Pancreatic cancer, a disease with a grim five-year survival rate of just 12%, claims over 60,000 new victims annually in the U.S. alone, and for smokers, the danger
In the ever-evolving landscape of pharmaceutical research, a staggering challenge persists: the need to uncover novel compounds that can tackle complex diseases with precision and efficacy, especially as resistance to existing drugs continues to grow. Scientists have long grappled with the
What if the very device connecting your home to the internet could also safeguard your health? Picture a typical morning: coffee brews in the kitchen, a laptop streams the latest news, and silently, the same WiFi router tracks heart rates with precision rivaling medical tools. This isn’t a distant
In a world where diabetes affects millions and places an immense strain on healthcare systems globally, a revolutionary approach to treatment has emerged from the Phase 3a ONWARDS 1–6 trials, showcasing the potential of Once-Weekly Insulin Icodec. This innovative therapy, distinct from the daily

What if a machine could save a life by seeing what even the sharpest human eye might miss? In the high-stakes world of heart health, where every second counts after a heart attack, artificial intelligence (AI) is stepping into the spotlight with groundbreaking potential. A recent study has revealed
Imagine a world where the development of life-saving drugs, a process that often stretches over a decade and costs billions of dollars, is condensed into just a few months with remarkable precision. This isn’t a distant dream but a tangible reality being shaped by NVIDIA through its innovative
Imagine a world where the smallest, most overlooked components of human cells hold the key to combating some of the most pressing health crises, such as obesity and aging. Mitochondrial microproteins, tiny yet powerful regulators within cells, are emerging as a groundbreaking focus in biomedical
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy