

Imagine a world where treatments for complex diseases are tailored to an individual’s unique genetic makeup, offering hope to patients who have long been out of options, and Alnylam Pharmaceuticals, a pioneer in RNA interference (RNAi) therapeutics, is turning this vision into reality by joining
In the rapidly evolving field of biomedical imaging, where cutting-edge technology meets the urgent demands of medical research, success often hinges on more than just technical expertise. Consider the challenge faced by many scientists transitioning from academic labs to industry roles: how does

What if the secret to managing blood sugar levels tomorrow morning lies in the choices made at tonight’s dinner table? For millions grappling with prediabetes, a condition marked by elevated glucose levels not yet at diabetic thresholds, this question is more than a thought experiment—it’s a

In an era where healthcare is increasingly driven by personalized solutions, the announcement of a pioneering role at a major medical institution captures attention as a sign of transformative progress in the field of medicine. Genomic medicine, which tailors treatments to an individual’s genetic

What if a simple blood test could reveal the exact weight-loss drug that works best for an individual, cutting through years of frustrating trial and error, and offering hope in a world where obesity affects over 650 million adults? The struggle to find effective treatment is real and deeply

Endometrial cancer, the most prevalent form of uterine cancer, continues to pose a significant public health challenge, particularly due to its disproportionate impact on certain demographic groups, with a growing incidence among postmenopausal women aged 55 to 64. This disease reveals stark
Imagine a world where cancer treatments can target tumors with pinpoint accuracy, sparing healthy tissues from harmful side effects, and bringing hope to millions. This vision is inching closer to reality with a groundbreaking technology developed by researchers at the University of Cambridge. The
Imagine stepping into a bustling concert venue where every inch is packed with enthusiastic fans, movement is slow, and personal space is nearly nonexistent, yet somehow, everything functions with a strange kind of order. This vivid scene mirrors the surprising reality of the cytoplasm inside the
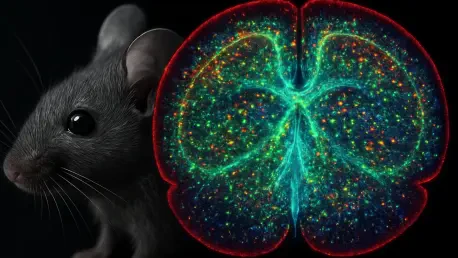
Imagine a world where scientists can peer into the intricate workings of a mouse’s brain as it scurries through a maze, forages for food, or interacts with its peers, all without restricting its natural movements, and witness in real time how neuronal processes drive behavior. This once-distant

Brazil is emerging as a formidable force in the global clinical research arena, thanks to the recent enactment of Law 14.784/14, a pivotal regulation designed to overhaul the nation’s approach to clinical trials. Spearheaded by ANVISA, Brazil’s health regulatory agency, this legislation targets
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy