

Imagine a world where smart contracts on blockchain platforms like Ethereum can seamlessly interact with real-world data—think weather updates, crop yields, or live market prices—without relying on potentially vulnerable centralized systems. Blockchain technology, celebrated for its trustless and

In an era where technology is revolutionizing every aspect of life, Medicare telehealth services have emerged as a critical resource for older Americans seeking accessible healthcare, especially during the COVID-19 pandemic when digital consultations via video calls or phone conversations became a

I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert whose deep knowledge of technology and innovation in the industry has positioned him as a leading voice in research and development. Today, we’re diving into the fascinating study of Maria Branyas Morera, who lived to the
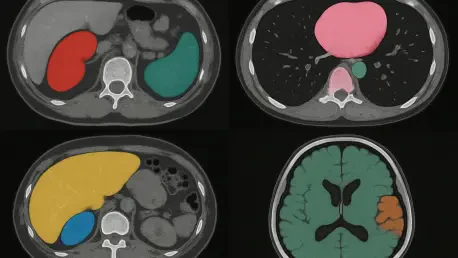
In the intricate world of medical research, imaging plays a pivotal role in uncovering insights into diseases and guiding life-saving treatments, yet the process of segmenting these images—manually delineating specific areas like tumors or organs—remains a daunting challenge that consumes countless
What if the key to unlocking life-saving treatments for diseases like cancer lies not in creating new drugs, but in perfecting how they reach their target? Every year, countless promising therapies fail due to poor delivery mechanisms, leaving patients and researchers grappling with unmet needs. At

Imagine a world where the arduous process of developing life-saving drugs, which often spans over a decade and costs billions of dollars, is dramatically shortened and made more cost-effective through cutting-edge technology. This vision is becoming increasingly tangible as artificial intelligence
Imagine a world where a routine blood test, combined with cutting-edge technology, could reveal the future trajectory of a spinal cord injury (SCI) within mere days of a patient arriving at a hospital, transforming uncertainty into actionable insight. This isn’t a distant dream but the reality of a

The pharmaceutical industry stands at a critical juncture, navigating a seismic shift from conventional distribution channels to direct-to-patient (DTP) models that promise to reshape how medications reach those in need. Once viewed as a tentative experiment to bypass intermediaries, DTP has
In a world where the race to develop life-changing therapies is accelerating at an unprecedented pace, biotech innovators are turning to artificial intelligence to redefine the boundaries of drug discovery, and Absci Corporation, a Seattle-based company listed on NASDAQ under the ticker ABSI,
In a world where cancer continues to challenge medical science, recent advancements offer a glimmer of hope for millions of patients grappling with this complex disease, and each year, countless individuals face diagnoses that turn their lives upside down. The urgency to develop effective,
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy