Imagine a world where the tiniest blood vessels in a brain can be studied in exquisite detail, revealing the hidden mechanisms that protect it from devastating disorders. A pioneering study from Florida Atlantic University (FAU) has achieved this feat by developing an advanced computer model that maps out blood flow in the mouse brain with unparalleled precision. Published in PLOS ONE, this research, conducted by experts from the College of Engineering and Computer Science and the FAU Sensing Institute (I-SENSE), dives into the intricate vascular system that sustains brain health. By focusing on how blood vessels adapt to shifting conditions, the study offers a window into cerebral autoregulation—a process critical for preventing conditions like stroke and Alzheimer’s disease. This breakthrough not only enhances scientific understanding but also sets the stage for transformative medical advancements.
Unveiling the Vascular Maze
Decoding the Mouse Brain’s Blood Network
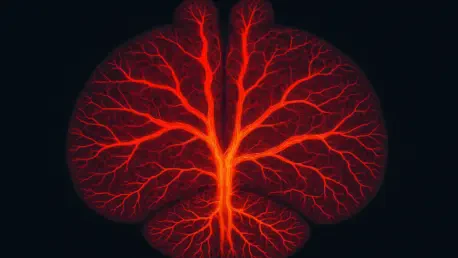
The FAU study centers on an elaborate network of blood vessels within the mouse brain, spanning from large arteries down to microscopic capillaries that are barely visible to the naked eye. At the core of this research are transitional zone (TZ) vessels, including penetrating arterioles, precapillary arterioles, and capillary sphincters. These intermediary structures bridge the gap between major arteries and tiny capillaries, playing a pivotal role in fine-tuning blood distribution. The computer model meticulously simulates how these vessels respond to fluctuations in blood pressure or brain activity, providing a detailed perspective on their dynamic behavior. Understanding TZ vessels is essential, as they appear to be the linchpin in maintaining a stable blood supply under varying physiological demands, a factor that could unlock new approaches to studying brain health.
Beyond the technical marvel of mapping these vessels, the study emphasizes their significance in the broader context of cerebral function. TZ vessels act as gatekeepers, adjusting their shape and resistance to ensure that oxygen and nutrients reach every corner of the brain, even when external conditions change dramatically. This adaptability is not just a biological curiosity; it’s a protective mechanism that safeguards against potential damage from sudden pressure spikes or drops. The FAU model captures these intricate adjustments, offering researchers a tool to explore scenarios that would be impossible to replicate in a living organism. This virtual representation marks a significant step forward in visualizing how the brain’s vascular architecture operates as a cohesive, responsive system.
Why Blood Flow Matters
A steady blood supply is the cornerstone of a healthy brain, serving as the lifeline that delivers oxygen and nutrients to support every thought and action. Disruptions in this flow, whether from blockages or sudden surges, are directly linked to severe neurological conditions such as stroke, traumatic brain injuries, and Alzheimer’s disease. The FAU research underscores how even minor imbalances at the microvascular level can cascade into major health crises if left unchecked. By modeling blood flow dynamics, scientists can now better understand the thresholds at which these disruptions become dangerous, paving the way for strategies to mitigate risks before they escalate into irreversible damage.
This focus on blood flow regulation also highlights a broader challenge in neuroscience: the brain’s vulnerability despite its sophisticated defenses. While larger arteries provide the bulk of blood transport, it’s the smaller vessels, particularly in the TZ, that make critical adjustments to maintain equilibrium during stress or heightened activity. The model developed by FAU researchers reveals how fragile this balance can be, especially when external factors push the system beyond its limits. Such insights are vital for developing interventions that could strengthen these defenses, potentially reducing the incidence of debilitating disorders through targeted therapies or preventative measures based on precise vascular behavior.
Innovation in Modeling
Simulating the Brain’s Defense System
The ingenuity of the FAU study lies in its integration of computational power with real biological data to create a model that mirrors the brain’s vascular responses with striking accuracy. Each vessel segment in this simulation is treated as an adjustable valve, capable of constricting or dilating in response to changes in blood pressure or flow demands. This approach allows researchers to observe how the system maintains cerebral autoregulation—the brain’s ability to stabilize blood flow despite external fluctuations. By validating the model’s predictions against actual biological measurements, the team has established a reliable tool that transcends the limitations of direct observation, offering a virtual lens into the brain’s protective mechanisms.
Equally impressive is the model’s capacity to replicate complex interactions between hemodynamics, the movement of blood, and vasodynamics, the physical changes in vessel structure. This dual focus captures the real-time interplay that governs how blood is distributed across the brain, even under challenging conditions. The simulation not only enhances understanding of normal function but also identifies potential breaking points where regulation fails, leading to stress on vessel walls or inadequate supply to brain tissues. Such detailed insights are invaluable for researchers aiming to bridge the gap between theoretical biology and practical medical applications, setting a new standard for how computational tools can inform health science.
Depth and Dynamics of Blood Flow
One of the standout revelations from the FAU model is its depiction of functional hyperemia, the process by which blood flow increases to meet the brain’s heightened demands during activity. This phenomenon varies significantly depending on a vessel’s location within the brain’s layered structure. In the outer regions, TZ vessels and sphincters take the lead in adjusting flow, ensuring that surface-level tissues receive adequate support. In contrast, deeper areas rely more heavily on penetrating arterioles to manage supply, reflecting a specialized division of labor within the vascular network. This depth-dependent behavior underscores the brain’s remarkable complexity and the model’s ability to capture such nuanced differences.
Further analysis through the model shows how these regional variations contribute to overall brain resilience. When one area experiences a surge in activity, the surrounding vascular system must compensate without disrupting balance elsewhere—a feat that requires precise coordination. The simulation reveals how different vessel types collaborate to achieve this, with TZ vessels often acting as the first line of response to redirect flow where it’s needed most. These findings provide a clearer picture of how the brain prioritizes resources during critical moments, offering potential clues for addressing conditions where this coordination falters, such as in neurodegenerative diseases or after trauma.
Key Discoveries and Implications
Phases of Pressure and Protection
Among the most compelling findings from the FAU research is the identification of four distinct phases in how the mouse brain responds to blood pressure changes. At very low pressures, blood flow drops below optimal levels, risking insufficient supply to vital tissues and compromising function. Within an ideal range, the system achieves a stable “sweet spot,” where flow remains consistent across a wide spectrum of pressures. However, beyond a critical threshold, control diminishes, leading to rapid flow increases that can strain or damage vessel walls. This loss of regulation is a key concern, as it may contribute to the onset of neurological issues over time, highlighting the importance of understanding these pressure boundaries.
The study also pinpointed TZ vessels as the primary regulators in navigating these phases, making minute adjustments to protect the brain from extremes. Despite their crucial role, these vessels have limits—endothelial cells lining them can only constrict so much before the system becomes vulnerable to uncontrolled flow. This vulnerability underscores the delicate nature of cerebral autoregulation and the potential consequences of pushing beyond physiological tolerances. By mapping out these phases, the model provides a framework for identifying at-risk conditions and developing interventions that could bolster the brain’s natural defenses against pressure-related stress.
Future Horizons in Brain Health
Looking ahead, the implications of this research extend far beyond academic curiosity, holding promise for real-world medical breakthroughs. Senior author Ramin Pashaie, PhD, emphasized that while healthy brains possess finely tuned systems for self-protection, even minor failures in these mechanisms can lead to significant consequences. The detailed insights from the model lay the groundwork for innovative diagnostic tools, such as non-invasive retinal imaging combined with artificial intelligence. This approach could detect early signs of Alzheimer’s disease by identifying corresponding changes in blood flow patterns between the brain and retina, offering a simple yet powerful method for early intervention.
Additionally, the potential to refine this model for application to human brain data opens exciting avenues for therapeutic development. The ability to simulate and predict vascular behavior could inform treatments for a range of neurological conditions, from stroke recovery to managing chronic disorders. As researchers continue to build on these findings, the collaboration between engineering, neuroscience, and technology promises to reshape how brain health challenges are approached. Ultimately, this work not only deepens the understanding of fundamental physiology but also points toward actionable solutions for some of society’s most pressing health concerns, reflecting a significant stride forward in the field.