A call from a doctor’s office following a routine mammogram can instantly create a cloud of anxiety, but the words “we need to take another look” often begin a journey into a frustrating gray area of medical uncertainty. For the nearly 50 percent of women with dense breast tissue, an inconclusive scan is an all-too-common reality, initiating a stressful cycle of follow-up appointments, additional imaging, and often, invasive biopsies that ultimately prove unnecessary. This diagnostic dilemma has long been a challenge in oncology, but a groundbreaking signal processing technology developed by researchers at Johns Hopkins University is poised to replace that uncertainty with definitive clarity, promising a future where a first look is the only look a patient needs.
The Agonizing Wait for Clarity in Breast Screening
The path of breast cancer screening is designed to be straightforward, yet for many, it is riddled with doubt. Mammography, the established gold standard, works by using low-dose X-rays to examine breast tissue. While effective for many, its diagnostic power diminishes significantly when faced with dense breast tissue, where fibrous and glandular tissue are more prevalent than fatty tissue. On a mammogram, both dense tissue and potential tumors appear white, making it incredibly difficult for radiologists to distinguish a dangerous mass from the normal, healthy background.
This ambiguity forces a move to the next diagnostic step: a follow-up ultrasound. However, this secondary screening method has its own set of limitations that perpetuate the cycle of uncertainty. The anxiety experienced by patients during this period is profound, as they are left to wait for a clear answer that current technology cannot always provide. This gap in diagnostic confidence not only takes a significant emotional toll but also places a substantial burden on the healthcare system through repeated testing.
When Standard Imaging Falls Short
Conventional ultrasound operates by sending sound waves into the breast and interpreting the echoes that bounce back. A simple, fluid-filled cyst—which is harmless—should allow sound waves to pass through it, creating a pure black image. A solid mass, which could be cancerous, reflects the waves and appears gray. The problem arises from a phenomenon known as “acoustic clutter.” In dense tissue, sound waves scatter chaotically, creating distortions that are similar to static on a radio.
This acoustic clutter can contaminate the image of a benign cyst, causing its black interior to appear speckled with gray. As a result, a harmless structure can visually mimic a potentially malignant solid mass. Radiologists, tasked with making a critical diagnosis based on this flawed information, are often left with no choice but to err on the side of caution. This frequently leads to a recommendation for a biopsy, a painful and invasive procedure, to physically determine the nature of the mass. The high rate of false positives from this process underscores a fundamental limitation in how we currently see inside the human body.
A Breakthrough in Seeing the Unseen
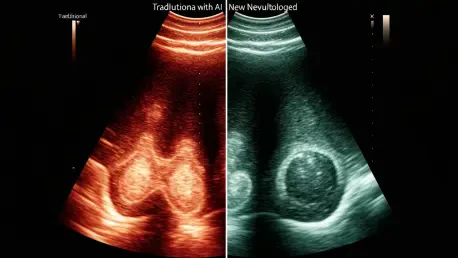
The innovation pioneered by a Johns Hopkins team, led by biomedical and electrical engineer Muyinatu “Bisi” Bell, represents a paradigm shift in how ultrasound data is interpreted. Crucially, the advancement is not in the physical ultrasound machine but in the software that processes the raw data it collects. Traditional ultrasound systems build an image based on the amplitude, or strength, of returning sound waves. Stronger signals create brighter pixels, while weaker ones are darker. This method, however, is highly susceptible to the distortions of acoustic clutter.
In contrast, the Johns Hopkins method is coherence-based. Instead of measuring signal strength, it analyzes the uniformity of the returning sound waves. Signals that reflect off a true anatomical structure are orderly and consistent, or “coherent.” Signals generated by random scatter and clutter are chaotic and “incoherent.” By intelligently filtering out this incoherent noise and focusing only on the unified signals, the new software constructs an image of astonishing clarity, effectively erasing the static that has long clouded diagnostic judgment.
A Leap in Accuracy and Confidence
The practical impact of this new approach was validated in a clinical study involving 132 patients, and the results were nothing short of transformative. When analyzing breast masses with conventional ultrasound, radiologists correctly identified their nature 67% of the time. When using the new coherence-based technology on the exact same scans, their accuracy surged to an impressive 96%. This dramatic improvement brings the diagnostic capability of ultrasound to a level of near-certainty.
Further enhancing its clinical utility, the system includes an integrated numerical scoring system. In addition to a clearer picture, the software assigns a quantitative score to a mass, providing an objective metric to supplement the radiologist’s visual interpretation. This feature helps to automate analysis and reduce “decision fatigue,” giving clinicians a data-driven tool to confirm their findings. The direct benefit for patients is immeasurable. As study co-author and radiologist Dr. Eniola Oluyemi noted, this enhanced certainty drastically reduces false positives, minimizes the need for painful follow-ups, and provides “increased peace of mind.”
Toward Instant Diagnosis and At Home Monitoring
The potential applications for this technology are only beginning to be explored. Researchers envision combining the coherence-based imaging with sophisticated artificial intelligence platforms. An AI trained on these ultra-clear images could learn to identify the signatures of benign and malignant masses with unparalleled precision, potentially creating a tool that offers an instantaneous diagnosis during a patient’s very first ultrasound appointment.
Looking further ahead, Dr. Bell imagines a future where this technology empowers individuals to take a more direct role in their own health. As ultrasound devices become more compact and affordable, her long-term vision includes integrating this intelligent software into simple, at-home scanners. In such a scenario, an individual performing a breast self-exam could use the device, and “a single number extracted from a coherence-based ultrasound image could tell whether or not a palpable breast lump is something to be concerned about,” transforming a moment of fear into one of empowered action.
The development of this coherence-based ultrasound processing marked a pivotal moment in medical imaging. By providing a clear and decisive way to differentiate between benign and malignant breast masses, the innovation addressed the long-standing clinical challenge of diagnostic uncertainty. This leap forward offered a pathway to reduce the physical and emotional burden on patients, who for too long endured the stress of inconclusive results and unnecessary procedures. The technology provided clinicians with a tool that transformed a subjective art of interpretation into a more objective, data-backed science, ultimately setting a new standard for confidence and care in breast health.