The path from discovering a suspicious breast lump to receiving a definitive diagnosis is often fraught with anxiety, culminating in a biopsy that, for a vast majority of individuals, confirms the lesion is benign. In the United States alone, an astonishing 75% to 80% of all breast biopsies yield non-cancerous results, a statistic that underscores a significant challenge in modern diagnostics. While biopsies remain the gold standard for confirmation, their high frequency for benign conditions contributes substantially to healthcare expenditures and imposes considerable physical and emotional distress on patients. This diagnostic uncertainty has spurred the development of new technologies aimed at refining the decision-making process. A breakthrough imaging method known as ultrasound-guided diffuse optical tomography is emerging as a powerful adjunctive tool, representing a major step toward a more precise, less invasive, and less stressful diagnostic pathway for breast cancer. This noninvasive technique promises to provide physicians with the critical information needed to confidently identify low-risk lesions, potentially sparing countless individuals from unnecessary procedures.
A New Lens on Breast Health
The Pioneering Innovation
The development of this advanced imaging technique was spearheaded by a multidisciplinary team at Washington University in St. Louis, driven by the shared goal of improving diagnostic accuracy in breast imaging. This pioneering work, led by Quing Zhu, the Edwin H. Murty Professor of Engineering, and Dr. Debbie Bennett, chief of breast imaging for WashU Medicine, focuses on creating detailed 3D images that reveal the functional characteristics of breast tissue. Unlike standard anatomical imaging, which primarily shows the structure of a lesion, this new method measures key physiological markers, such as blood vascular contrast and oxygen levels. These markers differ significantly between normal, benign, and cancerous tissues, offering deeper biological insight not available through conventional ultrasound alone. The technology is specifically designed to be used in conjunction with standard-of-care evaluations, acting as a crucial secondary check to help physicians more effectively triage low-risk breast lesions and confidently avoid biopsies unlikely to find cancer. This approach empowers clinicians with more comprehensive data, enhancing their ability to make more informed and personalized patient care decisions.
The collaboration between the McKelvey School of Engineering and the Mallinckrodt Institute of Radiology (MIR) was fundamental to translating this complex engineering concept into a practical clinical tool. This partnership highlights the importance of integrating advanced technological research with frontline medical expertise to address real-world healthcare challenges. The core objective was to bridge the diagnostic gap that often exists between an initial suspicious finding on an ultrasound and the definitive, yet invasive, step of a biopsy. By providing radiologists with a noninvasive way to assess a lesion’s physiological state, the technology aims to reduce the uncertainty that frequently leads to a biopsy recommendation “just to be safe.” The project’s significance was recognized with a nearly $2 million grant from the National Cancer Institute of the National Institutes of Health, which supported the large-scale clinical validation necessary to prove its efficacy. This backing underscores the national importance of developing solutions that can alleviate the burden of unnecessary medical procedures on both patients and the healthcare system.
The Science Behind the Scan
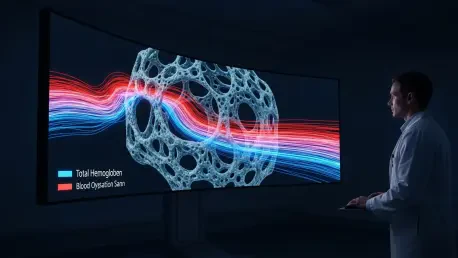
The operational principle of the technology is rooted in the unique way near-infrared light interacts with hemoglobin, the protein in red blood cells responsible for transporting oxygen. The system emits harmless near-infrared light into the breast tissue and measures how it is absorbed and scattered. Because hemoglobin is the primary absorber of this light in the breast, the system can create detailed maps of two key cancer biomarkers: total hemoglobin concentration and blood oxygen saturation. A higher-than-normal concentration of total hemoglobin is a strong indicator of increased blood flow and angiogenesis—the formation of new blood vessels. This process is a well-established hallmark of tumor growth, as malignant cells require a robust blood supply to fuel their rapid proliferation. By quantifying hemoglobin concentration, the scan provides a direct measure of this suspicious vascular activity, offering a clear, functional signal that a lesion may be cancerous. This goes beyond the structural information from an ultrasound, giving clinicians a view into the lesion’s underlying biology and its potential for malignancy.
Complementing the measurement of blood volume, the system’s ability to map blood oxygen saturation provides another critical layer of diagnostic information. A lower level of blood oxygen saturation within a lesion suggests it is metabolically hyperactive, consuming oxygen at a much faster rate than the surrounding healthy tissue. This high metabolic rate is another classic characteristic of a malignant tumor, which requires vast amounts of energy to sustain its growth and division. In contrast, benign lesions and normal tissue typically exhibit lower metabolic activity and thus higher oxygen saturation. During clinical trials, researchers confirmed these principles, finding that the total hemoglobin concentration in patients with malignant tumors was significantly higher than in those with benign lesions. Concurrently, the blood oxygen saturation was found to be significantly lower in cancerous lesions compared to their benign counterparts. Together, these two independent biomarkers provide a powerful and scientifically grounded basis for distinguishing between benign and malignant findings, enhancing diagnostic confidence.
From Lab to Clinic: The Evidence
A Rigorous Clinical Trial
To rigorously test the real-world clinical impact of this innovative technology, the research team designed and conducted a large-scale, double-blind clinical trial involving 226 patients, each presenting with a breast lesion that had a certain likelihood of malignancy. The study’s methodology was meticulously crafted to ensure an unbiased assessment of the new technique’s value. In the first phase of the trial, multiple participating radiologists evaluated each patient using only standard-of-care ultrasound, after which they made an initial recommendation on whether a biopsy was warranted. Following this standard assessment, they were provided with the additional data generated by the ultrasound-guided diffuse optical tomography system. With this new layer of functional information, which included the 3D maps of hemoglobin and oxygen saturation, the radiologists were asked to reconsider their initial biopsy decision. This double-blind approach was critical, as it prevented the optical tomography results from influencing the initial standard-of-care assessment, thereby allowing for a direct and objective comparison of decisions made with and without the new data.
The scale and design of this investigation marked a significant milestone, establishing it as the first study of its kind in the diffuse optical imaging field to involve such a large and diverse patient cohort and multiple practicing radiologists. This robust structure was essential for validating the technology’s utility across different clinical scenarios and operator interpretations, moving it from a promising concept to a clinically proven tool. The study cohort was representative of a typical clinical population, ultimately including 70 invasive carcinomas, seven ductal carcinomas in situ, nine high-risk lesions, and 140 benign lesions. By testing the system on a wide spectrum of findings, the researchers could accurately gauge its performance in distinguishing between conditions that require immediate intervention and those that can be safely monitored. The comprehensive nature of the trial provided the high-quality evidence needed to demonstrate not only the technology’s effectiveness but also its potential to be seamlessly integrated into existing clinical workflows to improve patient outcomes.
Significant and Safe Reduction
The results of the clinical trial were highly promising and demonstrated a clear clinical benefit. The integration of the optical tomography data with the radiologists’ standard classification assessment led to a remarkable 24.54% reduction in the number of recommended biopsies for lesions that were ultimately proven to be benign. This significant decrease underscores the technology’s ability to help clinicians confidently identify non-cancerous lesions that do not require an invasive procedure. Crucially, this reduction in unnecessary biopsies was achieved while maintaining an exceptionally high standard of patient safety. The study reported a false-negative rate below 2%, a critical safety metric that falls well within the stringent guidelines established by the American College of Radiology for standard-of-care imaging. This finding is vital, as it confirms that the technology can help avoid needless procedures without substantially increasing the risk of missing a true cancer diagnosis. The ability to safely and effectively rule out malignancy in a quarter of benign cases has profound implications for clinical practice.
The national impact of such a reduction could be transformative. With over one million breast biopsies performed annually in the United States, a nearly 25% reduction would translate to hundreds of thousands of patients avoiding the anxiety, discomfort, and cost associated with an invasive procedure each year. Professor Zhu emphasized this point, noting that the technology could have a profound effect on saving healthcare costs while simultaneously alleviating the significant stress that patients experience during the diagnostic process. The study’s success in demonstrating both a substantial decrease in benign biopsies and a strong safety profile provides compelling evidence for its adoption into routine clinical practice. By providing a more nuanced and biologically informed assessment of suspicious lesions, this adjunctive scan offers a clear path toward a more efficient, patient-centered, and cost-effective approach to breast cancer diagnosis, addressing a long-standing challenge in women’s health.
Beyond Biopsy Reduction: The Future
Further analysis of the clinical trial data revealed that the technology’s utility extended beyond simply reducing benign biopsies. The biomarkers it measures also correlated with the biological aggressiveness of the cancers that were detected. Specifically, the total hemoglobin concentration was found to be significantly higher in grade 3 cancerous lesions—which are more aggressive and faster-growing—than in grades 1 and 2. Conversely, blood oxygen saturation was significantly lower in these more aggressive tumors. This discovery suggested that the technology could potentially offer valuable insights into a tumor’s biology at the time of diagnosis, providing prognostic information that might one day help guide treatment planning. This capability represented a significant stride towards more personalized cancer diagnostics, where the initial imaging could do more than just detect the presence of cancer but also provide clues about its behavior.
With these successful results, the research team outlined a clear path forward. The immediate goals included further reducing the system’s manufacturing cost to ensure it could be an accessible tool for a wide range of clinical settings, not just major research hospitals. Simultaneously, efforts were focused on optimizing artificial intelligence tools to enable more efficient data processing and faster image reconstruction, which would streamline the workflow for busy radiologists. The ultimate objective was the commercialization of the technology to make it widely available for use at the bedside in clinics and imaging centers across the country. The publication of this research in late 2025 marked a pivotal moment, showcasing a validated technology poised to usher in a new era of more precise, less invasive, and more informative breast cancer care.An excellent and well-written text. I’ve made a few minor stylistic adjustments to enhance flow and clarity, consistent with standard American English conventions for scientific and medical writing.
The path from discovering a suspicious breast lump to receiving a definitive diagnosis is often fraught with anxiety, culminating in a biopsy that, for a vast majority of individuals, confirms the lesion is benign. In the United States alone, an astonishing 75% to 80% of all breast biopsies yield noncancerous results, a statistic that underscores a significant challenge in modern diagnostics. While biopsies remain the gold standard for confirmation, their high frequency for benign conditions contributes substantially to healthcare expenditures and imposes considerable physical and emotional distress on patients. This diagnostic uncertainty has spurred the development of new technologies aimed at refining the decision-making process. A breakthrough imaging method known as ultrasound-guided diffuse optical tomography is emerging as a powerful adjunctive tool, representing a major step toward a more precise, less invasive, and less stressful diagnostic pathway for breast cancer. This noninvasive technique promises to provide physicians with the critical information needed to confidently identify low-risk lesions, potentially sparing countless individuals from unnecessary procedures.
A New Lens on Breast Health
The Pioneering Innovation
The development of this advanced imaging technique was spearheaded by a multidisciplinary team at Washington University in St. Louis, driven by the shared goal of improving diagnostic accuracy in breast imaging. This pioneering work, led by Quing Zhu, the Edwin H. Murty Professor of Engineering, and Dr. Debbie Bennett, chief of breast imaging for WashU Medicine, focuses on creating detailed 3D images that reveal the functional characteristics of breast tissue. Unlike standard anatomical imaging, which primarily shows the structure of a lesion, this new method measures key physiological markers, such as blood vascular contrast and oxygen levels. These markers differ significantly between normal, benign, and cancerous tissues, offering deeper biological insight not available through conventional ultrasound alone. The technology is specifically designed to be used in conjunction with standard-of-care evaluations, acting as a crucial secondary check to help physicians more effectively triage low-risk breast lesions and confidently avoid biopsies unlikely to find cancer. This approach empowers clinicians with more comprehensive data, enhancing their ability to make more informed and personalized patient care decisions.
The collaboration between the McKelvey School of Engineering and the Mallinckrodt Institute of Radiology (MIR) was fundamental to translating this complex engineering concept into a practical clinical tool. This partnership highlights the importance of integrating advanced technological research with frontline medical expertise to address real-world healthcare challenges. The core objective was to bridge the diagnostic gap that often exists between an initial suspicious finding on an ultrasound and the definitive, yet invasive, step of a biopsy. By providing radiologists with a noninvasive way to assess a lesion’s physiological state, the technology aims to reduce the uncertainty that frequently leads to a biopsy recommendation “just to be safe.” The project’s significance was recognized with a nearly $2 million grant from the National Cancer Institute of the National Institutes of Health, which supported the large-scale clinical validation necessary to prove its efficacy. This backing underscores the national importance of developing solutions that can alleviate the burden of unnecessary medical procedures on both patients and the healthcare system.
The Science Behind the Scan
The operational principle of the technology is rooted in the unique way near-infrared light interacts with hemoglobin, the protein in red blood cells responsible for transporting oxygen. The system emits harmless near-infrared light into the breast tissue and measures how it is absorbed and scattered. Because hemoglobin is the primary absorber of this light in the breast, the system can create detailed maps of two key cancer biomarkers: total hemoglobin concentration and blood oxygen saturation. A higher-than-normal concentration of total hemoglobin is a strong indicator of increased blood flow and angiogenesis—the formation of new blood vessels. This process is a well-established hallmark of tumor growth, as malignant cells require a robust blood supply to fuel their rapid proliferation. By quantifying hemoglobin concentration, the scan provides a direct measure of this suspicious vascular activity, offering a clear, functional signal that a lesion may be cancerous. This goes beyond the structural information from an ultrasound, giving clinicians a view into the lesion’s underlying biology and its potential for malignancy.
Complementing the measurement of blood volume, the system’s ability to map blood oxygen saturation provides another critical layer of diagnostic information. A lower level of blood oxygen saturation within a lesion suggests it is metabolically hyperactive, consuming oxygen at a much faster rate than the surrounding healthy tissue. This high metabolic rate is another classic characteristic of a malignant tumor, which requires vast amounts of energy to sustain its growth and division. In contrast, benign lesions and normal tissue typically exhibit lower metabolic activity and thus higher oxygen saturation. During clinical trials, researchers confirmed these principles, finding that the total hemoglobin concentration in patients with malignant tumors was significantly higher than in those with benign lesions. Concurrently, blood oxygen saturation was found to be significantly lower in cancerous lesions compared to their benign counterparts. Together, these two independent biomarkers provide a powerful and scientifically grounded basis for distinguishing between benign and malignant findings, enhancing diagnostic confidence.
From Lab to Clinic: The Evidence
A Rigorous Clinical Trial
To rigorously test the real-world clinical impact of this innovative technology, the research team designed and conducted a large-scale, double-blind clinical trial involving 226 patients, each presenting with a breast lesion that had a certain likelihood of malignancy. The study’s methodology was meticulously crafted to ensure an unbiased assessment of the new technique’s value. In the first phase of the trial, multiple participating radiologists evaluated each patient using only standard-of-care ultrasound, after which they made an initial recommendation on whether a biopsy was warranted. Following this standard assessment, they were provided with the additional data generated by the ultrasound-guided diffuse optical tomography system. With this new layer of functional information, which included the 3D maps of hemoglobin and oxygen saturation, the radiologists were asked to reconsider their initial biopsy decision. This double-blind approach was critical, as it prevented the optical tomography results from influencing the initial standard-of-care assessment, thereby allowing for a direct and objective comparison of decisions made with and without the new data.
The scale and design of this investigation marked a significant milestone, establishing it as the first study of its kind in the diffuse optical imaging field to involve such a large and diverse patient cohort and multiple practicing radiologists. This robust structure was essential for validating the technology’s utility across different clinical scenarios and operator interpretations, moving it from a promising concept to a clinically proven tool. The study cohort was representative of a typical clinical population, ultimately including 70 invasive carcinomas, seven ductal carcinomas in situ, nine high-risk lesions, and 140 benign lesions. By testing the system on a wide spectrum of findings, the researchers could accurately gauge its performance in distinguishing between conditions that require immediate intervention and those that can be safely monitored. The comprehensive nature of the trial provided the high-quality evidence needed to demonstrate not only the technology’s effectiveness but also its potential to be seamlessly integrated into existing clinical workflows to improve patient outcomes.
Significant and Safe Reduction
The results of the clinical trial were highly promising and demonstrated a clear clinical benefit. The integration of the optical tomography data with the radiologists’ standard classification assessment led to a remarkable 24.54% reduction in the number of recommended biopsies for lesions that were ultimately proven to be benign. This significant decrease underscores the technology’s ability to help clinicians confidently identify noncancerous lesions that do not require an invasive procedure. Crucially, this reduction in unnecessary biopsies was achieved while maintaining an exceptionally high standard of patient safety. The study reported a false-negative rate below 2%, a critical safety metric that falls well within the stringent guidelines established by the American College of Radiology for standard-of-care imaging. This finding is vital, as it confirms that the technology can help avoid needless procedures without substantially increasing the risk of missing a true cancer diagnosis. The ability to safely and effectively rule out malignancy in a quarter of benign cases has profound implications for clinical practice.
The national impact of such a reduction could be transformative. With over one million breast biopsies performed annually in the United States, a nearly 25% reduction would translate to hundreds of thousands of patients avoiding the anxiety, discomfort, and cost associated with an invasive procedure each year. Professor Zhu emphasized this point, noting that the technology could have a profound effect on saving healthcare costs while simultaneously alleviating the significant stress that patients experience during the diagnostic process. The study’s success in demonstrating both a substantial decrease in benign biopsies and a strong safety profile provides compelling evidence for its adoption into routine clinical practice. By providing a more nuanced and biologically informed assessment of suspicious lesions, this adjunctive scan offers a clear path toward a more efficient, patient-centered, and cost-effective approach to breast cancer diagnosis, addressing a long-standing challenge in women’s health.
Beyond Biopsy Reduction: The Future
Further analysis of the clinical trial data revealed that the technology’s utility extended beyond simply reducing benign biopsies. The biomarkers it measures also correlated with the biological aggressiveness of the cancers that were detected. Specifically, the total hemoglobin concentration was found to be significantly higher in grade 3 cancerous lesions—which are more aggressive and faster-growing—than in grades 1 and 2. Conversely, blood oxygen saturation was significantly lower in these more aggressive tumors. This discovery suggested that the technology could potentially offer valuable insights into a tumor’s biology at the time of diagnosis, providing prognostic information that might one day help guide treatment planning. This capability represented a significant stride towards more personalized cancer diagnostics, where the initial imaging could do more than just detect the presence of cancer but also provide clues about its behavior.
With these successful results, the research team outlined a clear path forward. The immediate goals included further reducing the system’s manufacturing cost to ensure it could be an accessible tool for a wide range of clinical settings, not just major research hospitals. Simultaneously, efforts were focused on optimizing artificial intelligence tools to enable more efficient data processing and faster image reconstruction, which would streamline the workflow for busy radiologists. The ultimate objective was the commercialization of the technology to make it widely available for use at the bedside in clinics and imaging centers across the country. The publication of this research in late 2025 marked a pivotal moment, showcasing a validated technology poised to usher in a new era of more precise, less invasive, and more informative breast cancer care.