Imagine a world where a devastating condition like Parkinson’s disease (PD), which impacts millions globally with its debilitating tremors, stiffness, and slowed movements, can be detected long before symptoms disrupt daily life. This progressive neurodegenerative disorder stems from the loss of dopamine-producing neurons in a specific brain region called the substantia nigra, often leaving patients and clinicians grappling with uncertainty in early diagnosis. Historically, identifying PD has relied on observing physical signs during clinical exams, a process prone to ambiguity, especially when distinguishing it from similar movement disorders. Yet, groundbreaking strides in brain imaging technologies are revolutionizing this landscape, offering unprecedented clarity through direct visualization of brain changes, thus enhancing diagnostic precision and opening doors to earlier intervention.
These imaging advancements are rapidly shifting from mere supportive tools to essential components in managing PD. No longer confined to confirming what clinicians suspect, they provide concrete evidence of neurological alterations, such as dopamine depletion, that define the disease. The diversity of these methods—from established scans to experimental approaches—creates a multifaceted toolkit that addresses various aspects of PD pathology. This progress not only promises more accurate diagnoses but also fuels hope for personalized treatment plans tailored to individual brain profiles, potentially altering the trajectory of the disease.
Established Imaging Techniques
Dopamine Transporter Scan (DaTscan) and Its Role
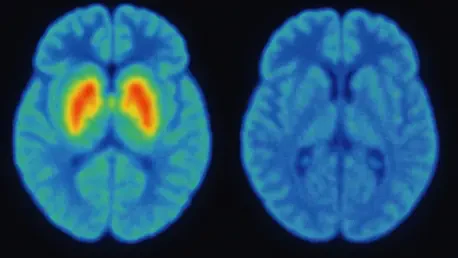
The Dopamine Transporter Scan, commonly known as DaTscan, stands as a cornerstone in the current arsenal of imaging tools for PD. This technique employs a radioactive tracer, Ioflupane 123I, which binds to dopamine transporters in the brain, revealing their density in the striatum—a region critical for movement control. A diminished signal in this area often points to PD, providing a vital clue for clinicians seeking to confirm a diagnosis. Widely available in clinical settings, DaTscan has become a go-to method due to its ability to offer objective evidence of dopamine loss, a hallmark of the disease. However, despite its utility, challenges persist, as many insurance providers do not cover it specifically for PD diagnosis, creating barriers to access for numerous patients who could benefit from this insight.
Beyond its diagnostic capabilities, DaTscan plays a significant role in differentiating PD from other conditions that mimic its symptoms, such as essential tremor. This distinction is crucial, as misdiagnosis can lead to inappropriate treatments and delayed care. While the scan’s results are often compelling, they are not standalone proof of PD, requiring integration with clinical assessments for a complete picture. Additionally, the use of a radioactive tracer raises concerns for some patients, though the exposure is minimal and generally considered safe. As a result, while DaTscan remains a pivotal tool, its limitations highlight the need for complementary methods to address gaps in accessibility and comprehensive evaluation.
PET Scans for Early Detection
Positron Emission Tomography, or PET scans, represent another powerful avenue for early detection of PD, with variants like FDG-PET and F-DOPA PET leading the charge. FDG-PET measures glucose metabolism, uncovering distinct patterns of brain activity associated with PD, while F-DOPA PET directly assesses dopamine production by tracking the uptake of a radiolabeled dopamine precursor. Both methods boast impressive accuracy, often achieving sensitivity and specificity rates near 95-100%, making them invaluable for identifying PD in its early stages and distinguishing it from other parkinsonian syndromes like multiple system atrophy. This precision is particularly critical when symptoms are subtle or overlap with other disorders, ensuring patients receive the correct diagnosis sooner.
Despite their strengths, PET scans come with notable drawbacks that temper their widespread adoption. The high cost of these procedures, coupled with the need for specialized equipment and expertise, limits their availability to major medical centers, often leaving rural or underserved populations without access. Furthermore, the use of radioactive tracers introduces a small but real risk of radiation exposure, a concern for repeated imaging over time. These factors underscore the importance of balancing the diagnostic benefits of PET scans with practical considerations, pushing the field to explore more cost-effective and safer alternatives that can deliver similar insights without the associated burdens.
Emerging Non-Invasive Methods
MRI Innovations and Accessibility
Magnetic Resonance Imaging (MRI) has long been a staple in medical diagnostics, but recent innovations are tailoring its application to PD with remarkable potential. Techniques like Neuromelanin-sensitive MRI (NM-MRI) focus on detecting the loss of neuromelanin, a pigment in dopamine neurons, while Quantitative Susceptibility Mapping (QSM) identifies iron deposits in the substantia nigra, both of which are linked to PD pathology. These non-invasive approaches offer a significant advantage by avoiding radiation, making them safer for repeated use. With MRI machines widely available in hospitals worldwide, these methods hold promise for broader implementation, potentially reaching patients who lack access to more specialized scans.
However, the journey from research to routine clinical use for these MRI techniques is still underway. Currently, both NM-MRI and QSM remain largely experimental, with variability in image quality and interpretation posing challenges to standardization. Studies have shown impressive results, such as NM-MRI’s ability to distinguish PD patients from healthy controls with high sensitivity, but consistent protocols across different facilities are needed to ensure reliability. As these hurdles are addressed, the accessibility of MRI-based methods could democratize advanced diagnostics, providing a clearer view of brain changes without the financial or logistical barriers often associated with other imaging technologies.
Transcranial Sonography (TCS) as a Cost-Effective Tool
Transcranial Sonography (TCS) emerges as a surprisingly affordable and portable option for early PD screening, utilizing ultrasound to visualize brain structures. This technique often reveals a brighter, or hyperechogenic, substantia nigra in PD patients compared to healthy individuals, offering a potential early indicator of the disease. With reported accuracy rates around 88%, TCS stands out for its simplicity and low cost, making it particularly appealing in resource-limited settings where advanced imaging equipment might be scarce. Its ease of use also means that it can be performed in outpatient clinics, reducing the need for patients to travel to specialized centers.
Yet, the limitations of TCS cannot be overlooked, as its lack of specificity means it struggles to differentiate PD from other conditions with similar brain changes. This reduces its reliability as a standalone diagnostic tool, often necessitating confirmation with other methods. Additionally, the quality of results can vary depending on the operator’s expertise and the equipment used, highlighting a need for better standardization. Despite these challenges, TCS represents a valuable stepping stone in expanding access to early detection, particularly for communities that might otherwise face delays in diagnosis due to economic or geographic constraints.
Future Horizons in PD Imaging
Targeting Core Pathology with Alpha-Synuclein Imaging
One of the most exciting frontiers in PD diagnostics lies in the development of imaging agents designed to detect alpha-synuclein, a misfolded protein central to the disease’s pathology. The accumulation of this protein in the brain is a defining feature of PD, driving neuron loss long before symptoms manifest. While still in preclinical stages, early research into PET ligands for alpha-synuclein shows promise, with studies in animal models demonstrating the ability to visualize these aggregates. If successful, this approach could transform diagnosis by targeting the root cause rather than downstream effects like dopamine loss, potentially enabling detection years earlier than current methods allow.
The implications of alpha-synuclein imaging extend far beyond diagnosis, offering a window into the disease’s progression and response to therapies aimed at clearing these protein clumps. However, significant obstacles remain, including the need to develop safe and effective tracers that can cross the blood-brain barrier and bind specifically to alpha-synuclein without interference from other proteins. Clinical trials are still on the horizon, and widespread use may be years away. Nevertheless, the potential to directly address the core mechanism of PD fuels optimism, positioning this technology as a possible game-changer in how the disease is understood and managed.
Advanced Research Tools
In the realm of experimental imaging, techniques like Diffusion Tensor Imaging (DTI), resting-state functional MRI (fMRI), and Magnetoencephalography (MEG) are pushing boundaries by exploring brain connectivity and activity. DTI maps white matter pathways, revealing structural disruptions in PD, while fMRI assesses how brain networks communicate during rest, shedding light on functional impairments. MEG, on the other hand, captures brain wave activity with high precision, offering clues about neural dynamics. Though primarily research tools at this stage, these methods provide nuanced insights, particularly into non-motor symptoms such as cognitive decline, which often precede or accompany motor issues in PD.
The future clinical application of these advanced tools holds immense potential, especially for creating comprehensive profiles of PD’s impact across different brain systems. For instance, understanding altered connectivity through fMRI could inform strategies to address mood disorders or memory problems linked to PD. However, their complexity, high cost, and the need for specialized expertise limit current use to academic and research settings. As technology evolves and becomes more accessible, integrating these insights with other diagnostic methods could offer a fuller picture of the disease, paving the way for tailored interventions that address both motor and non-motor challenges.
Integrative Approaches
Combining Modalities for Comprehensive Diagnosis
A growing trend in PD imaging is the integration of multiple modalities to create a more detailed and accurate diagnostic framework. By combining structural data from MRI techniques like NM-MRI or QSM with functional insights from PET scans or DaTscan, clinicians can capture a multidimensional view of brain changes. This approach addresses the limitations of individual tests, as no single method fully encapsulates the complex pathology of PD. For example, pairing evidence of neuromelanin loss with dopamine transporter reduction could strengthen diagnostic certainty, while also providing clues about disease severity and progression over time.
This integrative strategy extends beyond diagnosis to monitoring treatment outcomes, offering a way to assess how therapies impact various aspects of brain function and structure. Research into these combinations is still developing, with challenges in aligning data from different imaging platforms and ensuring consistent interpretation. Yet, the push toward such comprehensive approaches aligns with the broader movement in medicine toward precision and personalization. As protocols for combining modalities are refined, they could redefine PD management, ensuring that each patient receives a diagnosis and care plan uniquely suited to their brain’s specific profile.
Paving the Way for Personalized Medicine
The ultimate vision for brain imaging in PD is to enable truly personalized medicine, where diagnostic tools not only confirm the presence of the disease but also guide individualized treatment strategies. Combining imaging modalities allows for a deeper understanding of how PD manifests differently across patients—some may show pronounced dopamine loss, while others exhibit significant protein accumulation or connectivity issues. This tailored insight could inform decisions about which medications or interventions might work best, as well as predict potential complications like cognitive decline, enabling proactive measures to improve quality of life.
Achieving this level of personalization requires overcoming significant hurdles, including the high costs and technical demands of multi-modal imaging, as well as ensuring that such approaches are scalable to diverse healthcare settings. Collaboration between researchers, technology developers, and policymakers will be essential to address these barriers, alongside efforts to validate combined imaging strategies in large-scale clinical trials. Despite these challenges, the trajectory of integrating imaging data points to a future where PD care is as unique as the patients themselves, maximizing the impact of every therapeutic step through precise, data-driven decisions.
Looking Ahead to Transformative Solutions
Reflecting on the strides made in brain imaging for Parkinson’s disease, it’s evident that the field has witnessed remarkable progress in providing clearer, more reliable diagnostic pathways. Tools like DaTscan and PET scans have laid a robust foundation, while emerging methods such as NM-MRI and alpha-synuclein imaging push the boundaries of what is possible. Each step forward tackles unique facets of PD’s complex pathology, from dopamine loss to protein buildup, enhancing the ability to distinguish this condition from similar disorders. The dedication to overcoming limitations like cost and accessibility underscores a commitment to equitable healthcare advancements.
Moving into the next phase, the focus should pivot toward actionable solutions that bridge the gap between cutting-edge research and everyday clinical practice. Prioritizing the standardization of promising techniques like TCS and advanced MRI methods will ensure consistent results across diverse settings. Additionally, accelerating clinical trials for integrative imaging approaches can hasten their adoption, while advocacy for broader insurance coverage could alleviate financial burdens. Investing in training for healthcare providers to handle these sophisticated tools will further embed them into routine care. These steps, taken collectively, promise to refine early detection and personalized treatment, ultimately reshaping the landscape of PD management for future generations.