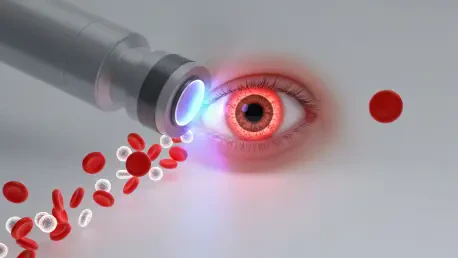
The routine procedure of drawing blood remains one of the most common yet consistently invasive practices in modern medicine, a necessary discomfort for millions seeking crucial health insights. But a new frontier in diagnostics is emerging, one that promises to replace the needle with a simple, non-invasive scan of the eye. This transformative vision is now one step closer to reality through a strategic business development by DiagnosTear Technologies Inc., a company specializing in rapid eye-based diagnostics. The firm has signed a non-binding term sheet with two of Israel’s most prominent research institutions, Sheba Impact, the technology transfer office for Sheba Medical Center, and Ramot, representing Tel Aviv University. This collaboration is set to propel a groundbreaking technology from the laboratory to the forefront of patient care, potentially revolutionizing how basic health metrics are gathered in clinical and emergency settings worldwide and marking a significant shift toward painless, accessible medical analysis.
A Glimpse into the Future of Diagnostics
At the heart of this partnership is the exclusive licensing of “CLARIFY,” a pioneering eye imaging technology co-developed by the Israeli research hubs. This platform is designed to perform a complete blood count without a single drop of blood. The system operates by employing advanced spectral imaging to meticulously analyze the tiny blood vessels on the surface of the sclera, the white part of the eye. The captured data is then processed through sophisticated, proprietary algorithms that can accurately measure a host of critical blood components. This includes quantifying red and white blood cells, assessing hemoglobin-related indicators, and even identifying specific inflammatory signatures. The technology effectively bypasses the need for a physical blood draw, offering a painless and immediate alternative. Remarkably, the feasibility of this innovative approach was first demonstrated during a space mission that was focused on developing non-invasive methods for blood assessment, highlighting its potential for use in remote and challenging environments where traditional medical procedures are impractical.
Forging a Path from Lab to Clinic
The recent signing of the non-binding term sheet represented a foundational framework for a future definitive agreement. The objective of the parties involved was to finalize a comprehensive research and licensing deal that would establish a structured clinical development plan and a clear pathway to commercialization. Dr. Shimon Gross, the CEO of DiagnosTear, highlighted that the partnership with these world-class research institutions was intended to significantly accelerate the clinical validation and eventual market release of the CLARIFY platform. The ultimate goal was to transform routine patient care by providing a versatile diagnostic tool with broad applications. The technology held promise for a variety of medical fields, including the management of chronic conditions, rapid assessment in emergency settings, and routine health screenings. This strategic move aimed to leverage the combined expertise of academic innovation and corporate development to bring a truly disruptive medical device to patients and healthcare providers.