Imagine a diagnosis so devastating that it offers a median survival of just one year, primarily striking children and young adults with little hope for effective intervention. Diffuse midline glioma with an ### K27M mutation, an ultra-rare and aggressive brain cancer, affects roughly 2,000
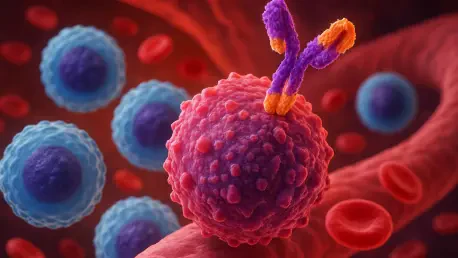
What happens when a relentless cancer, deemed incurable, meets a groundbreaking therapy that defies the odds? For the 15,000 Americans diagnosed annually with follicular lymphoma—a slow-growing but persistent form of non-Hodgkin lymphoma—this question is no longer hypothetical. A stunning phase 3
I'm thrilled to sit down with Ivan Kairatov, a distinguished biopharma expert with a wealth of experience in research and development, particularly in the intersection of technology and innovation in healthcare. Today, we're diving into a groundbreaking advancement in the field of neurosurgery: a

In the intricate world of biopharmaceuticals, where the journey from drug discovery to patient delivery is fraught with challenges, the roles of Clinical Operations (ClinOps) and Medical Affairs (MA) stand out as indispensable. ClinOps serves as the operational backbone, meticulously managing

In the fast-evolving landscape of obesity treatment, where pharmaceutical companies race to deliver groundbreaking solutions, Viking Therapeutics emerged with high hopes for their oral weight loss drug, VK2735. Touted as a convenient alternative to injectable therapies dominating the market, this
What happens when the very food meant to nourish us becomes a silent carrier of deadly threats? Each year, an estimated 600 million people worldwide fall ill from foodborne illnesses, with 420,000 losing their lives to pathogens that have grown resistant to traditional defenses, posing a growing