What happens when a single microscopic misstep can trigger a cascade of catastrophic consequences like cancer or genetic disorders? Every day, trillions of cells in the human body divide, splitting their genetic material with astonishing precision to sustain life, but when this process falters, the results can be devastating. A groundbreaking discovery by scientists at the Ruđer Bošković Institute in Zagreb, Croatia, has unveiled a hidden layer of complexity in cell division, centering on a protein called CENP-E. This revelation challenges decades of biological understanding and promises to reshape approaches to some of humanity’s most pressing health challenges.
The Heart of Life’s Engine
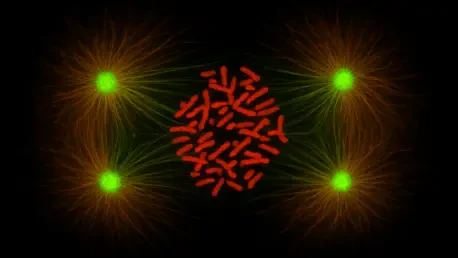
Cell division, known as mitosis, lies at the core of growth, healing, and reproduction. Each time a cell divides, it must ensure that its chromosomes—the carriers of genetic code—are split evenly between two new cells. Errors in this process, often tied to improper chromosome alignment, are linked to severe conditions such as infertility and developmental defects. More alarmingly, these mistakes are a hallmark of cancer, where cells multiply uncontrollably with abnormal genetic content. The urgency to decode the mechanisms of mitosis has never been greater, as cancer remains a leading global cause of death, claiming millions of lives annually.
This is where the recent findings on CENP-E come into sharp focus. Long regarded as a mere mechanical player in moving chromosomes during division, this protein has now emerged as a critical regulator. The significance of this shift cannot be overstated: understanding CENP-E’s true function offers a potential key to addressing the root causes of diseases that plague countless individuals. This discovery is not just a scientific milestone; it is a beacon of hope for innovative therapies that could save lives.
Redefining a Cellular Giant
For years, biology textbooks painted CENP-E as a motor protein, a kind of cellular tugboat dragging chromosomes to their proper positions during mitosis. However, the team at the Ruđer Bošković Institute has shattered this simplistic view with evidence published in a prestigious journal. Their research shows that CENP-E operates not by force but by finesse, stabilizing the delicate initial connections between chromosomes and microtubules—the tiny cellular tracks that guide alignment. Without this stabilizing role, chromosomes fail to line up at the cell’s center, risking catastrophic division errors.
Beyond this primary function, the study reveals CENP-E’s intricate partnership with other cellular components, notably Aurora kinases. These proteins act as gatekeepers, sending signals to break incorrect attachments and prevent mistakes. CENP-E steps in as a mediator, softening these harsh signals to allow just enough stability for proper connections to form at the right moment. This balancing act is crucial for ensuring that each daughter cell inherits the correct genetic blueprint, highlighting a level of precision previously unimagined in cellular mechanics.
Another layer of complexity emerges with the role of centromeres, the central hubs on chromosomes where microtubules attach. Far from being passive anchors, centromeres actively communicate with CENP-E through feedback loops, fine-tuning the alignment process. This cooperative dynamic transforms the understanding of mitosis from a brute mechanical operation into a sophisticated symphony of regulation, with CENP-E conducting the critical movements.
Voices from the Lab
The researchers behind this paradigm shift express both awe and determination in their findings. Dr. Kruno Vukušić, a key figure in the study, emphasizes the real-world stakes: “This discovery pinpoints mechanisms tied directly to disease, paving the way for groundbreaking medical approaches.” His perspective underscores the urgency of translating lab results into tangible benefits for patients grappling with conditions rooted in faulty cell division.
Professor Iva Tolić, co-leader of the project, highlights the technological edge that fueled their success. “By harnessing advanced computational modeling, supported by state-of-the-art facilities at the University of Zagreb, the team visualized CENP-E’s regulatory role with unprecedented clarity,” she notes. Their work, backed by significant funding from the European Research Council, exemplifies how cutting-edge tools and collaborative efforts can challenge long-held scientific dogmas, pushing the boundaries of knowledge.
The excitement extends beyond the research team to the broader scientific community, where experts see this as a catalyst for rethinking cellular biology. The meticulous experiments, combined with innovative simulations, lend robust credibility to the findings. This consensus signals a turning point, urging scientists worldwide to reexamine other assumed truths in the field with fresh eyes and renewed vigor.
From Lab to Life: What This Means for Medicine
The implications of redefining CENP-E’s role stretch far beyond academic curiosity, offering concrete pathways for medical innovation. Chromosome segregation errors, a direct outcome of disrupted mitosis, are a driving force behind many cancers. With CENP-E now identified as a regulatory linchpin, pharmaceutical researchers have a new target for developing drugs that could interrupt tumor growth by manipulating this protein’s function or its interaction with Aurora kinases. Such therapies might one day halt the relentless spread of cancerous cells.
Educational systems must also adapt to this new reality. Biology curricula, long rooted in outdated models of CENP-E as a motor protein, need revision to reflect its regulatory nature. Educators are encouraged to integrate these findings into teaching materials, emphasizing the importance of timing and control over mere physical movement in cellular processes. This shift ensures that future generations of scientists approach the study of life with a more nuanced perspective.
Looking ahead, the scientific community can build on this foundation by leveraging technology to explore other hidden regulatory networks in cell division. Combining experimental data with powerful simulations, as the Croatian team did, could uncover additional targets for combating diseases. This approach promises to accelerate discoveries, turning fundamental insights into therapies that address the cellular roots of human suffering.
A Legacy of Discovery
Reflecting on this monumental achievement, it becomes clear that the work at the Ruđer Bošković Institute marks a defining moment in biological science. The revelation of CENP-E as a regulator rather than a mechanical mover rewrites a chapter of cellular understanding that had stood unchallenged for decades. It illuminates the intricate dance of proteins and structures within dividing cells, showcasing a precision that inspires both wonder and resolve among researchers.
As a next step, the global scientific community is urged to prioritize funding and collaboration to further map the regulatory landscape of mitosis. Biotech innovators are encouraged to fast-track the development of CENP-E-targeted treatments, potentially transforming cancer care. Meanwhile, educators take up the mantle to reshape biology lessons, ensuring students grasp the elegance of cellular regulation. This collective effort holds the promise of not just understanding life at its smallest scale, but of bending it toward healing and hope for millions.