Imagine a world where life-saving treatments for cancer, autoimmune diseases, and metabolic disorders no longer require hours of intravenous infusions in a clinical setting, allowing patients to administer their medications at home with a quick injection, reclaiming time and autonomy. This scenario is inching closer to reality thanks to a revolutionary protein drug delivery platform developed by researchers at Stanford University. This technology tackles one of the most persistent challenges in modern medicine: delivering high-concentration protein therapeutics in a stable, injectable form. By redefining how these drugs are formulated and administered, this innovation promises to transform healthcare delivery and patient outcomes.
Unveiling the Core Technology
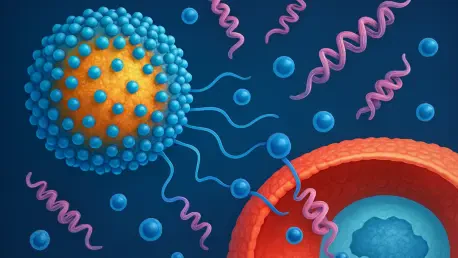
At the heart of this platform lies a novel approach to stabilizing protein drugs at unprecedented concentrations. Traditional methods often limit protein formulations to low concentrations to prevent aggregation or viscosity issues, necessitating lengthy IV infusions. Stanford’s solution bypasses these constraints by encasing proteins in a protective coating that maintains their integrity, even at levels exceeding 500 mg/mL. This leap forward not only reduces treatment duration but also opens the door to self-administration, a significant shift from clinic-dependent care.
The technology’s adaptability stands out as a defining feature. Unlike many delivery systems tailored to specific drugs, this platform demonstrates versatility across various protein types. Tests on albumin, human immunoglobulin, and a monoclonal antibody for COVID have shown consistent stability under diverse conditions, including temperature fluctuations and freeze-thaw cycles. Such broad applicability suggests that the system could support a wide range of biologics, addressing unmet needs in multiple therapeutic areas.
Innovative Features Under the Microscope
Protective Coating with MoNi Polymer
Central to the platform’s success is a unique polyacrylamide copolymer known as MoNi. This material forms a glass-like shield around protein molecules, characterized by a high glass transition temperature that keeps it solid even at elevated temperatures. This protective layer prevents the proteins from clumping together, a common issue that renders high-concentration formulations unusable and potentially harmful due to immune reactions.
The brilliance of MoNi lies in its ability to preserve the structural integrity of delicate biologics. By acting as a barrier against aggregation, it ensures that the proteins remain functional during storage and administration. This innovation addresses a critical pain point in pharmaceutical development, where stability often limits the practicality of protein-based drugs, paving the way for more robust therapeutic options.
Spray Drying for Precision Formulation
Another cornerstone of this technology is the spray drying process used to create the coated protein particles. In this method, proteins are aerosolized into tiny droplets, mixed with the MoNi polymer, and dried into a fine powder. Each particle emerges encased in a smooth, spherical coating, reminiscent of a candy shell, which facilitates easy suspension in a liquid carrier for injection.
This technique achieves what was once thought impossible: delivering ultra-high concentrations through standard syringes or autoinjectors. The smooth texture and uniform shape of the particles allow for seamless flow through tiny needles, eliminating the risk of clogging or uneven dosing. As a result, patients could potentially receive powerful doses in seconds rather than hours, a transformative step for chronic disease management.
Performance Metrics and Real-World Potential
The performance of this delivery system has been rigorously evaluated in preclinical settings, with results indicating exceptional stability and injectability. Formulations have withstood extreme conditions without degradation, a testament to the durability of the MoNi coating. Such resilience is crucial for ensuring that drugs remain effective during transportation and storage, particularly in regions with limited access to refrigeration.
Beyond technical achievements, the platform’s impact on healthcare delivery cannot be overstated. By enabling at-home injections, it reduces the burden on medical facilities and cuts down on costs associated with clinic visits. For patients with chronic conditions requiring frequent treatments, this translates to greater independence and improved quality of life, aligning with the industry’s shift toward patient-centered care.
The technology’s commercialization is already underway, with a local startup securing licensing rights to bring it to market. This step signals confidence in its scalability and real-world applicability. As development progresses from the current year to 2027, ongoing refinements are expected to further optimize its performance, potentially expanding its reach to even more complex biologics.
Challenges on the Horizon
Despite its promise, the path to widespread adoption is not without obstacles. Regulatory approval remains a significant hurdle, as extensive clinical trials are needed to confirm the safety and efficacy of these high-concentration formulations. Ensuring that the MoNi polymer poses no long-term risks to patients will be a key focus of these studies, requiring meticulous evaluation.
Scaling production also presents technical challenges. While spray drying is a well-established process in pharmaceuticals, adapting it for mass production of coated protein particles may encounter unforeseen complexities. Researchers are actively working to address these issues, refining the manufacturing process to meet the demands of a global market without compromising quality.
Additionally, integrating this platform into existing healthcare systems will require education and infrastructure support. Clinicians and patients alike must be trained on the new administration methods, and supply chains must adapt to handle these specialized formulations. Overcoming these logistical barriers will be essential to realizing the full potential of this innovation.
Reflecting on a Transformative Milestone
Looking back, Stanford’s protein drug delivery platform stood as a pivotal advancement in medical technology. Its ability to stabilize high-concentration protein therapeutics and enable simple injections marked a turning point in treatment accessibility. The journey from lab to preclinical success highlighted a commitment to addressing long-standing challenges in drug administration.
Moving forward, the focus should shift to accelerating clinical validation and fostering collaborations between researchers, regulators, and industry stakeholders. Streamlining the approval process while maintaining rigorous safety standards will be crucial. Additionally, investment in patient education programs can ensure smooth adoption of at-home injection systems.
Ultimately, the next steps involve exploring applications beyond currently tested proteins, potentially unlocking treatments for conditions once deemed untreatable due to formulation barriers. By continuing to innovate and adapt, this platform could redefine therapeutic landscapes, offering hope to millions worldwide through more efficient and empowering medical solutions.