The significant disparity in survival rates between early and late-stage breast cancer diagnoses has long propelled the search for more effective and frequent screening technologies beyond the established annual mammogram. The development of portable breast ultrasound represents a significant advancement in medical imaging and preventative healthcare. This review will explore the evolution of this technology, its key features, performance metrics, and the impact it has had on addressing critical gaps in breast cancer screening. The purpose of this review is to provide a thorough understanding of the technology, its current capabilities, and its potential future development.
The Dawn of Accessible Breast Imaging
The emergence of portable breast ultrasound technology is a direct response to a critical limitation in current screening protocols: the inability to detect aggressive cancers that develop between scheduled mammograms. These “interval cancers” account for a substantial portion of all breast cancer cases and are often more dangerous, underscoring the urgent need for a method of frequent monitoring. While traditional ultrasound is an effective imaging tool, its utility for routine screening is severely hampered by its reliance on bulky, expensive equipment and the requirement for highly trained technicians, creating significant accessibility barriers, especially in remote or underserved communities.
This new class of portable device aims to dismantle these barriers by leveraging miniaturization and user-centric design. By condensing the core components of an ultrasound machine into a compact and affordable system, it brings advanced diagnostic capabilities out of the specialized hospital setting. The goal is to empower individuals, particularly those at high risk, with a tool for regular self-monitoring. This shift has the potential to fundamentally alter the landscape of preventative care, moving from a reactive to a proactive model and enabling the detection of tumors at their earliest, most treatable stages.
Core Technology and System Architecture
Miniaturized Probe and 3D Imaging Array
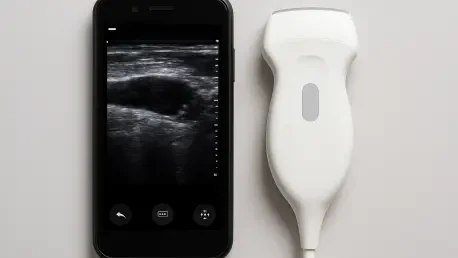
At the heart of this innovation is a compact ultrasound probe, ingeniously designed to deliver high-resolution, three-dimensional images of breast tissue. Slightly smaller than a deck of cards, the probe features a unique empty square array of transducers. This configuration is pivotal, as it allows the device to capture a wide-angle view without the need for complex mechanical scanning components, a common feature in larger, traditional systems. This design streamlines the imaging process, enabling the capture of a complete breast volume from just a few positions.
A significant advantage of this design is its ability to produce anatomically accurate, undistorted images. Unlike conventional ultrasound probes that must be pressed firmly into the tissue, which can cause deformation, this device rests gently on the skin. This non-invasive contact ensures that structures like cysts and potential tumors are imaged in their true size and location. The result is a more reliable 3D map of the breast tissue, providing clinicians with a clearer and more precise view for diagnosis and monitoring.
Low Power Cost Effective Processing Module
Complementing the innovative probe is an equally groundbreaking data acquisition motherboard, which serves as the system’s electronic brain. This processing module, barely larger than a smartphone, is the key to the system’s portability and affordability. Constructed from commercially available electronic components, its production cost is remarkably low, estimated at around three hundred dollars. This stands in stark contrast to the tens of thousands of dollars required for the complex electronics that power hospital-grade ultrasound machines.
Furthermore, the system’s design prioritizes energy efficiency. The motherboard operates on a minimal 5V DC power supply, allowing it to be powered by a simple battery pack or a standard wall adapter. This low power consumption liberates the technology from the constraints of dedicated power infrastructure, making it suitable for use in a wide variety of settings, from a local clinic to a patient’s home. This combination of low cost and low power is a critical step toward democratizing access to advanced medical imaging.
Advancements Beyond First Generation Prototypes
The current portable ultrasound system represents a significant leap forward from earlier iterations, such as the wearable patch developed by the same research team three years ago. While that first-generation prototype was a pioneering step toward continuous monitoring, it was tethered to a conventional, refrigerator-sized ultrasound machine for image processing. This dependency severely limited its practicality and portability, confining its use to clinical research environments.
The latest model rectifies these shortcomings by integrating a self-contained, miniaturized processing unit, making the entire system truly portable and independent. Another crucial enhancement is its ability to generate complete, gapless images of the entire breast. The previous patch design occasionally left small gaps in imaging coverage, creating a risk of missing small tumors. The new probe design ensures comprehensive scanning, significantly improving its diagnostic reliability and moving the technology closer to becoming a viable clinical tool.
Real World Application and Clinical Validation
Addressing the Interval Cancer Detection Gap
The primary clinical application for this portable ultrasound system is to fill the dangerous detection gap left by annual mammography. The technology provides a practical means for frequent monitoring, empowering patients and clinicians to detect fast-growing interval cancers that can emerge and advance in the months between scheduled screenings. The five-year survival rate for breast cancer is nearly one hundred percent when detected at its earliest stage but drops dramatically to around twenty-five percent for cancers discovered at advanced stages.
By making frequent screening accessible and affordable, this device has the potential to shift the diagnostic timeline significantly. For high-risk individuals, the ability to perform regular checks could lead to diagnoses at Stage 0 or 1, dramatically improving prognoses and reducing the need for aggressive treatments. This paradigm shift from annual screening to more continuous monitoring represents a powerful new strategy in the fight against breast cancer.
Performance in Initial Human Trials
The system’s efficacy was successfully demonstrated in initial human trials, providing strong validation of its design and capabilities. During a test on a 71-year-old subject with a history of breast cysts, the device accurately identified and imaged the cysts, which were just a few millimeters in diameter. It successfully generated a high-resolution, three-dimensional map of the breast tissue down to a depth of fifteen centimeters.
These promising results have paved the way for more extensive clinical validation. The research team is now advancing to larger-scale trials at the MIT Center for Clinical and Translational Research and Massachusetts General Hospital. These trials will be crucial for gathering the comprehensive data needed to further refine the technology and prove its safety and effectiveness across a diverse patient population, a critical step toward regulatory approval.
Challenges and Hurdles to Widespread Adoption
Navigating Clinical Trials and Regulatory Pathways
Despite its promising initial results, the journey from a functional research prototype to a widely available medical device is fraught with challenges. The foremost hurdle is the rigorous process of clinical validation and regulatory approval. The technology must undergo extensive testing in large-scale clinical trials to definitively prove its diagnostic accuracy, reliability, and safety when compared to existing standards of care.
Successfully navigating this process requires substantial funding, time, and collaboration with medical institutions. The researchers must compile a robust body of evidence to satisfy regulatory bodies like the Food and Drug Administration (FDA). This process is designed to protect patients and ensure that any new medical device is both safe and effective for its intended use, representing a critical but lengthy step before the technology can reach the public.
Ensuring User Friendliness for At Home Application
Beyond regulatory hurdles, a significant design challenge lies in making the device intuitive and reliable enough for at-home use by individuals without medical training. For the system to be effective as a self-monitoring tool, users must be able to operate it correctly and consistently to acquire high-quality images. This requires a user interface that is simple to understand and provides clear, actionable feedback.
Achieving this level of user-friendliness involves overcoming several technical obstacles. The device must be robust enough to withstand regular use, and its software must be sophisticated enough to guide the user through the scanning process. Ensuring that a layperson can consistently capture diagnostically useful images without the direct supervision of a healthcare professional is a complex but essential goal for realizing the technology’s full potential in preventative healthcare.
The Future of Personal and Preventative Screening
Next Generation Miniaturization and Smartphone Integration
The development roadmap for this technology envisions even greater levels of portability and integration. A primary near-term goal is to shrink the data processing system from its current smartphone-sized form factor to the size of a fingernail. This radical miniaturization would allow the entire processing unit to be seamlessly integrated directly into the probe itself.
This advancement would enable the device to connect directly to a standard smartphone, which would serve as the interface for controlling the scan and viewing the resulting images. The creation of a truly pocket-sized, smartphone-powered ultrasound system would represent the ultimate in accessibility, placing a powerful diagnostic tool directly into the hands of patients and primary care providers anywhere in the world.
AI Assisted Guidance and Wearable Sensor Development
Looking further into the future, researchers are exploring the integration of artificial intelligence to enhance the system’s usability and diagnostic power. A key development will be an AI-driven smartphone application designed to guide users in placing the probe correctly on the breast. This virtual assistant would analyze the image quality in real-time and provide feedback, ensuring optimal coverage and reliable results even for untrained users.
The ultimate vision is to evolve this technology into a fully wearable sensor, perhaps integrated into a bra, that could provide continuous, passive monitoring for individuals at the highest risk of developing breast cancer. Such a device would automatically and regularly scan the breast tissue, using AI algorithms to detect any suspicious changes over time and alerting the user and their clinician at the earliest possible sign of a problem. This would represent the pinnacle of personalized, preventative medicine.
Summary and Final Assessment
The development of this portable breast ultrasound system represented a convergence of innovative engineering and a clear clinical need. The technology successfully integrated a miniaturized probe with a cost-effective, low-power processing module, overcoming the primary barriers of size, cost, and accessibility that limited traditional ultrasound. Its design demonstrated a tangible advancement over earlier prototypes by achieving a fully self-contained architecture and gapless, three-dimensional imaging.
Initial human trials validated the system’s core capabilities, proving its ability to generate high-resolution images of breast tissue and accurately identify small structures. While the path to widespread adoption required navigating significant regulatory and user-design challenges, the technology’s potential was undeniable. It stood as a powerful example of how targeted innovation could fundamentally change the approach to preventative healthcare, offering a future where early cancer detection was more accessible, frequent, and personalized.