
Imagine dining out with friends, eagerly awaiting a meal labeled "gluten-free," only to face the lingering fear of hidden gluten triggering severe health issues, a daily reality for millions with celiac disease or non-celiac gluten sensitivity. Cross-contamination and mislabeling remain pervasive
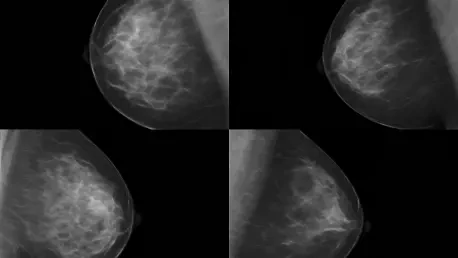
I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, particularly in innovative technologies shaping the future of healthcare. Today, we’re diving into a groundbreaking study from UCLA Health on the impact of regular
In a world where the race to develop life-changing therapies is accelerating at an unprecedented pace, biotech innovators are turning to artificial intelligence to redefine the boundaries of drug discovery, and Absci Corporation, a Seattle-based company listed on NASDAQ under the ticker ABSI,

The healthcare sector in the United States is at a critical juncture as private equity (PE) firms tighten their grip on hospitals, igniting fierce debate over the consequences for patient care and raising serious concerns about the balance between profit and well-being. With over $1 trillion
In a world still grappling with the aftermath of a global health crisis that recorded over 700 million cases and 7 million deaths, the role of COVID-19 vaccines stands as a testament to human ingenuity and scientific progress. These vaccines, developed at an unprecedented pace through years of
In a world where cancer continues to challenge medical science, recent advancements offer a glimmer of hope for millions of patients grappling with this complex disease, and each year, countless individuals face diagnoses that turn their lives upside down. The urgency to develop effective,