Mantle cell lymphoma (MCL), a rare yet aggressive form of blood cancer, affects approximately one in every 100,000 individuals annually, falling within the broader category of lymphoma, which ranks as the sixth most common cancer worldwide. Despite its relative rarity, the disease poses a significant challenge due to its resistance to long-term treatment and its complex genetic foundations. A recent study from the Centre for Genomic Regulation (CRG) in Barcelona, published in Nucleic Acids Research, has unveiled a transformative perspective on how specific genetic alterations propel MCL’s progression. This research introduces the concept of genome rewiring, a mechanism that extends far beyond isolated gene changes, revealing a sweeping impact on cellular behavior. By exploring these findings, this article aims to illuminate the intricate ways in which chromosomal errors drive this devastating condition, offering a glimpse into potential breakthroughs for future therapies and diagnostic approaches that could redefine patient care.
Unraveling the Genetic Errors in MCL
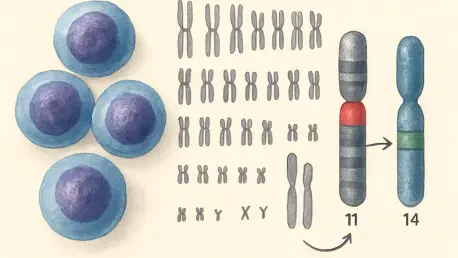
The foundation of MCL’s development often lies in a specific chromosomal translocation between chromosomes 11 and 14, a genetic error where segments of DNA break and reattach in abnormal configurations. This event relocates a powerful regulatory component known as the IGH enhancer from chromosome 14 to a position near the CCND1 gene on chromosome 11. In healthy B cells, the IGH enhancer plays a vital role in amplifying antibody production, but in the context of MCL, it abnormally boosts CCND1 activity, triggering relentless cell division—a hallmark of cancer. While this interaction has been recognized for some time, the depth of its consequences remained underestimated until now. This translocation serves as a critical starting point for understanding the broader genetic chaos that unfolds, setting the stage for a deeper investigation into how such errors reshape the cellular landscape in ways that sustain malignancy.
Beyond the immediate effect on CCND1, the implications of this chromosomal swap challenge traditional views of genetic disruptions in cancer. The latest findings suggest that the influence of the IGH enhancer is not confined to a single gene or even a small cluster of nearby genetic elements. Instead, it acts as a catalyst for widespread changes, altering the behavior of numerous genetic components in a manner previously unrecognized. This revelation underscores the necessity of looking beyond obvious genetic targets to grasp the full scope of damage inflicted by such translocations. It also raises important questions about how these alterations sustain the aggressive nature of MCL, pushing researchers to reconsider the mechanisms long thought to drive this disease. The complexity of these interactions highlights the urgent need for innovative approaches to both study and combat this relentless form of lymphoma.
Discovering the Vast Reach of Genetic Impact
A striking revelation from the study is the sheer scale of genetic disruption caused by the translocation, with the IGH enhancer affecting over 50 genes across a staggering 50 million base pairs on chromosome 11—roughly 7% of the chromosome’s total genetic content. This far-reaching impact creates a domino effect, where changes in one area reverberate through a significant portion of the genome, amplifying the potential for cancerous growth. Dr. Renée Beekman, the lead author of the research, described the magnitude of this discovery as astonishing, noting that it uncovers a host of previously overlooked genetic players in MCL’s progression. Such a broad sphere of influence suggests that the disease’s complexity is rooted in a network of interactions, far exceeding the narrow focus on breakpoint-adjacent genes that dominated earlier studies.
This expansive genetic influence opens up a wealth of new possibilities for therapeutic intervention, as each affected gene represents a potential target for treatment. The identification of such a large number of implicated genes shifts the paradigm of how MCL is approached, moving away from single-gene solutions to a more holistic view of genomic behavior. It also emphasizes the importance of advanced genetic profiling in understanding the full extent of damage caused by translocations. As researchers begin to map out these interactions, the hope is to pinpoint critical nodes within this network that could be disrupted to halt the disease’s advance. This discovery not only broadens the scientific understanding of MCL but also lays the groundwork for developing more tailored and effective strategies to combat its relentless progression, potentially improving outcomes for patients facing this challenging diagnosis.
Exploring the Spatial Dynamics of DNA
Central to understanding the widespread genetic impact in MCL is the role of DNA’s three-dimensional structure within cells, which organizes genetic material into intricate loops that bring distant segments into close proximity. When the IGH enhancer is translocated to chromosome 11, it integrates into an existing loop, positioning itself to exert control over multiple genes simultaneously. This spatial arrangement amplifies its influence, allowing it to supercharge the activity of genes that are already partially active rather than awakening dormant ones. Such a selective effect adds a layer of nuance to the enhancer’s role, suggesting that its impact may vary depending on the cellular context or developmental stage, a factor that could explain differing outcomes in patients with similar genetic errors.
The significance of DNA folding in this process cannot be overstated, as it reveals how physical architecture dictates genetic function in ways that linear models of DNA cannot capture. This three-dimensional perspective offers a new lens through which to view chromosomal translocations, highlighting the importance of spatial relationships in driving disease. It also suggests that therapeutic strategies might one day target these structural arrangements to disrupt harmful enhancer activity. By focusing on the loops and folds that facilitate genetic interactions, scientists could potentially mitigate the enhancer’s ability to influence multiple genes at once. This insight marks a critical step forward in decoding the complex mechanisms of MCL, providing a foundation for future research into how spatial dynamics contribute to cancer and how they might be manipulated to restore normal cellular function.
Advancing Research with Cutting-Edge Tools
To uncover these groundbreaking insights, the research team employed state-of-the-art CRISPR technology to replicate the chromosomal translocation in healthy B cells within a controlled laboratory environment. This innovative approach, spearheaded by co-author Dr. Roser Zaurin, enabled experiments that would be infeasible with patient-derived tissues due to ethical and technical limitations. By creating an early disease model through engineered cells, the scientists gained a unique window into the initial stages of genome rewiring, observing firsthand how the translocation triggers widespread genetic changes. This method not only provided clarity on the mechanisms driving MCL but also established a powerful platform for testing hypotheses about genetic interactions in a way that mirrors real-world conditions.
The use of CRISPR in this context represents a significant advancement in cancer research, offering a precision tool to dissect the intricate effects of genetic errors. Unlike traditional methods that rely on observational data from patient samples, this engineered model allows for controlled manipulation of specific genetic elements, yielding clearer insights into cause-and-effect relationships. Such an approach could accelerate the pace of discovery, enabling researchers to test potential interventions before moving to clinical stages. The success of this methodology in studying MCL also holds promise for other cancers driven by similar genetic mechanisms, potentially broadening its impact across oncology. By leveraging these cutting-edge tools, the scientific community moves closer to unraveling the complexities of genetic diseases, paving the way for more effective and personalized medical solutions.
Charting a Path for Future Therapies and Diagnostics
The implications of this research extend far into the realm of clinical application, as the identification of dozens of affected genes vastly expands the pool of potential drug targets for MCL. This broadened list offers renewed hope for developing innovative treatments that address the disease’s multifaceted nature, moving beyond the limitations of current therapies that often fail to achieve lasting remission. By targeting multiple genetic components influenced by the IGH enhancer, future drugs could disrupt the interconnected network that sustains cancer growth, potentially leading to more durable outcomes for patients. This shift in focus toward a wider array of targets marks a promising direction for overcoming the challenges posed by MCL’s aggressive and resistant profile.
Equally compelling is the potential for early detection strategies stemming from these findings, as suggested by Dr. Beekman. Profiling the epigenetic patterns of at-risk B cells could reveal dangerous gene activity before MCL fully manifests, allowing for intervention at a stage when the disease might be more manageable. Such a proactive approach could revolutionize how this lymphoma is diagnosed, shifting the emphasis from treatment to prevention in high-risk populations. The ability to identify and monitor subtle genetic changes before they escalate offers a powerful tool for improving survival rates. As research progresses, these dual prospects of novel therapies and preemptive diagnostics highlight the transformative potential of understanding genome rewiring, setting a hopeful trajectory for managing a disease that has long defied effective solutions.