Imagine a world where the body’s own cleanup crew could be trained to target and eliminate harmful cells, such as those driving cancer or autoimmune disorders, with pinpoint accuracy. This isn’t a distant dream but a reality being shaped by groundbreaking research into a protein-based tool called Crunch (Connector for Removal of Unwanted Cell Habitat). Developed by scientists at a leading research institute, this innovation harnesses the natural waste removal system of the human body to address some of the most challenging diseases. The significance of such a tool lies in its potential to offer less invasive, highly precise treatments compared to current methods.
The purpose of this FAQ article is to demystify Crunch and explore its implications for medical science. Key questions surrounding its mechanism, applications, and future potential will be addressed, providing clarity on how this tool could transform disease management. Readers can expect to gain a comprehensive understanding of the technology, its benefits, and the challenges that lie ahead in bringing it to clinical use.
This content will cover the basics of how Crunch operates, its testing outcomes, and the broader impact it may have on therapeutic approaches. By delving into specific aspects through targeted questions, the aim is to equip readers with actionable insights into a promising advancement in precision medicine. Whether curious about cancer treatments or autoimmune solutions, this article serves as a guide to understanding a cutting-edge development.
Key Questions or Key Topics Section
What Is Crunch and How Does It Work?
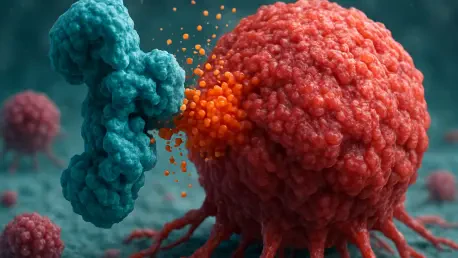
Crunch represents a novel protein-based therapeutic tool designed to target and remove harmful living cells that contribute to diseases like cancer and autoimmune conditions. Unlike traditional treatments that often kill cells directly, this tool ingeniously repurposes the body’s natural process of efferocytosis, where immune cells called phagocytes clear out dead cells. By modifying a protein known as Protein S, scientists have created Crunch to detect specific surface markers on unwanted living cells, signaling phagocytes to engulf and destroy them as if they were already dead.
The importance of this mechanism lies in its ability to mimic a natural immune response while applying it to living cells that cause harm. This approach avoids the collateral damage often seen with aggressive therapies, focusing instead on precision. Customizable sensors within Crunch allow it to identify unique proteins on target cells, ensuring that only the intended cells are marked for removal, which is a significant leap forward in therapeutic specificity.
Evidence from preclinical studies highlights the effectiveness of this design. In controlled experiments, Crunch successfully tricked the immune system into clearing specific cancer cells and overactive immune cells without disrupting healthy tissues. This innovative strategy underscores a shift toward leveraging inherent bodily functions for disease treatment, potentially reducing side effects associated with more invasive methods.
What Diseases Can Crunch Potentially Treat?
The scope of Crunch’s application spans a wide range of conditions characterized by harmful or unnecessary cells. Primarily, it shows promise in tackling cancer, where it targets malignant cells expressing distinct surface proteins, marking them for elimination by the immune system. Additionally, autoimmune disorders like lupus, where overactive immune cells attack healthy tissues, have been identified as key areas for intervention, with Crunch reducing disease symptoms in preclinical models.
This versatility stems from the tool’s customizable nature, allowing sensors to be tailored to different cell types based on their unique markers. Such adaptability positions Crunch as a platform technology, capable of addressing multiple health challenges beyond just oncology or autoimmunity. The potential to integrate with existing therapeutic frameworks, such as antibody-based treatments, further broadens its applicability across medical fields.
Supporting data from animal studies reinforces these possibilities. In tests involving mice, Crunch not only diminished cancer cell populations but also alleviated harmful immune activity in lupus models, showcasing measurable improvements in health outcomes. These results suggest that with further refinement, Crunch could become a cornerstone in managing complex diseases that currently lack effective, targeted solutions.
How Does Crunch Differ from Existing Therapies Like CAR-T Cell Therapy?
A distinguishing feature of Crunch compared to therapies like CAR-T cell treatment is its simplicity and method of action. While CAR-T therapy involves extracting a patient’s blood cells, modifying them in a lab to attack specific targets, and reinfusing them—a process that is both complex and costly—Crunch offers a more streamlined approach. It can potentially be administered through a simple injection, eliminating the need for extensive laboratory procedures.
This difference is crucial in terms of accessibility and scalability. By harnessing the body’s existing phagocytes without requiring external cell modification, Crunch minimizes logistical challenges and reduces the risk of severe immune reactions often associated with CAR-T treatments. The focus on using natural immune processes also suggests a safer profile, as it avoids introducing heavily engineered cells into the body.
Insights from researchers involved in Crunch’s development indicate a vision of integrating it with current therapeutic strategies for enhanced outcomes. Unlike direct cell-killing methods that can lead to widespread tissue damage, Crunch acts as a sophisticated labeling system, ensuring precision. This unique mechanism, backed by successful preclinical results, highlights its potential to complement or even replace more invasive options in the therapeutic landscape.
What Are the Current Challenges and Future Prospects for Crunch?
Despite its promising capabilities, Crunch faces several hurdles before it can be widely implemented in clinical settings. One primary challenge is ensuring its safety and efficacy across diverse patient populations, as preclinical success in controlled environments does not always translate to real-world scenarios. Additionally, scalability in production and customization of sensors for various diseases remain areas requiring significant refinement.
The importance of overcoming these obstacles cannot be overstated, as they directly impact Crunch’s transition from lab to bedside. Ongoing research efforts, starting this year and projected over the next few years, aim to address these issues by optimizing the tool’s design and testing it in more complex models. Ensuring that Crunch can be reliably tailored to individual patient needs without compromising safety is a key focus of these studies.
Looking ahead, the prospects for Crunch are encouraging, with potential to redefine precision medicine. Researchers express cautious optimism about its role in a broader therapeutic ecosystem, where it could be adapted for numerous conditions through sensor modifications. As advancements continue, Crunch may pave the way for treatments that are not only effective but also less burdensome, marking a significant step forward in disease management.
Summary or Recap
Crunch stands out as a transformative protein-based tool that leverages the body’s natural waste removal system to target harmful cells in diseases such as cancer and lupus. This FAQ has explored its innovative mechanism, which uses customized sensors to mark unwanted cells for elimination by phagocytes, distinguishing it from more invasive therapies like CAR-T cell treatment. The precision and adaptability of Crunch highlight its potential to address a wide array of medical conditions with minimal side effects.
Key takeaways include the tool’s ability to simplify treatment delivery through possible injection-based administration and its successful outcomes in preclinical studies, where it reduced disease symptoms in animal models. The challenges of safety, scalability, and real-world application remain critical areas of focus, yet the ongoing research efforts signal a commitment to overcoming these barriers. Crunch’s integration with existing therapeutic strategies further amplifies its promise as a versatile platform.
For those seeking deeper exploration, resources on immunology and protein engineering, available through academic journals and medical research databases, provide valuable context. Additionally, staying updated on advancements in precision medicine can offer further insights into how tools like Crunch evolve. This summary encapsulates the core aspects of a groundbreaking development poised to influence future treatment paradigms.
Conclusion or Final Thoughts
Reflecting on the journey of Crunch, it is evident that this protein-based tool marks a pivotal moment in therapeutic innovation, offering a glimpse into how natural immune processes can be harnessed for targeted disease treatment. Its ability to address complex conditions like cancer and autoimmune disorders with precision has sparked significant interest among researchers and medical professionals alike. The successful preclinical outcomes have laid a strong foundation, inspiring confidence in its potential.
As a next step, attention turns to bridging the gap between research and clinical application, with efforts focused on rigorous testing and refinement to ensure safety and efficacy. Stakeholders are encouraged to support and follow these developments, recognizing the transformative impact Crunch could have on patient care. Exploring collaborations with medical institutions and staying informed about trial results become essential actions for those invested in advancing healthcare solutions.
Ultimately, the story of Crunch prompts a broader consideration of how emerging technologies can be integrated into everyday medical practice. Individuals and communities alike are urged to think about the implications of such advancements on personal health strategies, advocating for access to cutting-edge treatments. This reflection serves as a call to remain engaged with innovations that promise to reshape the landscape of disease management for the better.