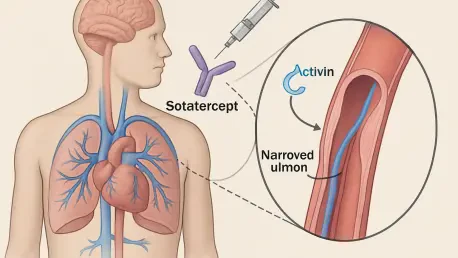
Pulmonary arterial hypertension (PAH), a life-threatening condition characterized by dangerously high blood pressure in the lung arteries, has long posed significant challenges for patients and clinicians alike due to its progressive nature and limited treatment options. Enter sotatercept, a biologic drug marketed as Winrevair, which has recently captured attention for its potential to revolutionize early intervention in PAH management. Groundbreaking clinical trials have unveiled striking results, particularly for patients diagnosed within their first year, suggesting that this innovative therapy could dramatically alter disease trajectories. With morbidity and mortality rates remaining alarmingly high for PAH sufferers, the emergence of sotatercept offers a beacon of hope, promising not only symptom relief but also a chance to prevent severe deterioration. This article explores the compelling findings from recent studies, delving into how this drug might reshape treatment approaches and improve quality of life for those battling this debilitating condition.
Unveiling Sotatercept’s Potential in Early Intervention
The HYPERION study, a phase 3, double-blind, placebo-controlled trial, has brought sotatercept into the spotlight as a transformative option for newly diagnosed PAH patients. When integrated with standard therapies within the first year of diagnosis, this biologic drug demonstrated a staggering 76% reduction in the risk of health deterioration, encompassing worsening symptoms, diminished exercise capacity, and unexpected hospitalizations. Such compelling evidence led to the trial’s early termination on ethical grounds, as researchers found it unjustifiable to withhold the drug from the placebo group given its undeniable efficacy. This outcome emphasizes sotatercept’s capacity to act swiftly and effectively, providing a critical window for intervention that could redefine how early-stage PAH is managed in clinical settings. The results signal a potential shift in focus toward rapid therapeutic action to curb the disease before it escalates to more severe stages.
Beyond the raw numbers, the significance of HYPERION lies in its unique emphasis on patients at the onset of their PAH journey, distinguishing it from prior studies like STELLAR and ZENITH, which targeted those with longer disease histories or higher risk profiles. Early intervention with sotatercept appears to help maintain a lower risk status, potentially staving off the long-term decline that often accompanies delayed treatment. This approach challenges traditional strategies that frequently address PAH only after it has advanced, when options become less effective and outcomes more dire. By prioritizing treatment shortly after diagnosis, sotatercept could prevent patients from reaching critical thresholds of disease progression, offering a proactive rather than reactive framework. This shift underscores the urgent need to rethink diagnostic and therapeutic timelines to maximize patient benefits in the earliest phases of this condition.
How Sotatercept Redefines PAH Treatment Mechanisms
Sotatercept stands out from conventional PAH treatments by targeting a distinct biological pathway, specifically activin signaling proteins implicated in the pathological thickening of pulmonary arteries. This thickening forces the heart to exert excessive effort to pump blood to the lungs, a hallmark of PAH that drives its debilitating effects. Unlike traditional therapies that primarily focus on symptom alleviation or blood vessel dilation, sotatercept offers a potential disease-modifying impact by addressing an underlying cause of arterial changes. This innovative mechanism positions the drug as a pioneering force in a field desperate for novel solutions, potentially altering the fundamental approach to managing this chronic condition. Such a targeted strategy could lead to more sustainable outcomes, providing a foundation for therapies that go beyond temporary relief to tackle the root issues driving PAH progression.
While the therapeutic promise of sotatercept is evident, its clinical profile also includes considerations of safety and tolerability that are crucial for widespread adoption. The HYPERION trial reported remarkably low hospitalization rates for worsening PAH symptoms among the treatment group, with fewer than 2% affected compared to 8.8% in the placebo arm, highlighting a strong protective effect against severe outcomes. However, minor side effects such as nosebleeds and spider veins were noted, though these did not significantly detract from the drug’s overall benefits. These manageable drawbacks suggest that while sotatercept is not without challenges, its advantages in preventing critical health events far outweigh the milder adverse reactions. This balance of efficacy and safety reinforces the drug’s potential to become a cornerstone in early PAH treatment, offering a viable option for patients who might otherwise face limited choices in managing their condition.
Evolving Perspectives on PAH Management
Insights from the HYPERION trial, supported by esteemed investigators like Dr. Vallerie V. McLaughlin and Dr. Victor M. Moles from the University of Michigan Medical School, point to a growing consensus that sotatercept could fundamentally change PAH treatment protocols. There is a palpable push within the medical community toward early and assertive intervention, aiming to alter the disease’s trajectory before irreversible damage occurs. This perspective aligns with broader medical trends favoring proactive care over reactive responses, particularly for chronic and progressive diseases like PAH where timing can be everything. The advocacy for integrating sotatercept into early treatment plans reflects a recognition that delaying effective therapy may compromise long-term patient outcomes, urging a reevaluation of how soon and how aggressively this condition should be addressed in clinical practice.
Additionally, the approval of sotatercept by the U.S. Food and Drug Administration for adult PAH patients marks a significant milestone in the acceptance of biologic therapies for complex diseases with few effective treatments. Its success when combined with existing standard therapies suggests a future where personalized, combination approaches become the norm, targeting specific disease mechanisms for optimal results. This trend toward tailored treatment strategies could herald a new era in PAH care, where patients benefit from therapies designed to complement each other, addressing both symptoms and underlying causes in a holistic manner. As biologic drugs like sotatercept gain traction, they pave the way for innovative management practices that prioritize individual patient needs, potentially setting a precedent for how other challenging conditions are approached in the medical field.
Looking Ahead to a New Era in PAH Care
Reflecting on the journey of sotatercept through clinical evaluation, the HYPERION trial marked a pivotal moment by establishing the drug as a powerful tool in early PAH treatment, slashing the risk of disease worsening and reducing hospitalization rates dramatically. Its novel mechanism targeting activin signaling provided a fresh perspective on managing a condition long plagued by limited options. Minor side effects were observed but did not overshadow the profound impact on patient outcomes. As the medical community embraced these findings, the push for early intervention gained momentum, reshaping how PAH was approached in its initial stages. Moving forward, the focus should center on integrating sotatercept into standard care protocols swiftly after diagnosis, ensuring access for newly diagnosed patients to prevent progression. Continued research into optimizing combination therapies and monitoring long-term effects will be crucial to solidify its role, offering a pathway to sustained improvements in quality of life for those affected by this challenging disease.