I’m thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, particularly in cutting-edge technologies and innovations in the industry. Today, we’re diving into a groundbreaking advancement in cancer treatment—a new formulation of the chemotherapy drug paclitaxel developed by researchers at the University of Arizona. In this conversation, we’ll explore how this innovative approach tackles the challenges of traditional chemotherapy, the science behind its unique delivery system, and the potential it holds for transforming cancer care and beyond.
Can you start by telling us about the new formulation of paclitaxel your team has developed and what inspired this research?
Absolutely, I’m excited to share this with you. Our team at the University of Arizona has created a novel formulation called Paclitaxome, which reimagines how paclitaxel—a widely used chemotherapy drug—can be delivered to tumors. The inspiration came from a critical need to address the limitations of current paclitaxel treatments, like Taxol and Abraxane. These drugs, while effective, often cause significant toxicity and don’t always reach the tumor in sufficient amounts. We wanted to improve both the precision of delivery and the drug’s staying power in the body, ultimately aiming to enhance outcomes for patients with cancers like breast and pancreatic cancer.
What sets this new approach apart from the traditional methods of delivering chemotherapy drugs like paclitaxel?
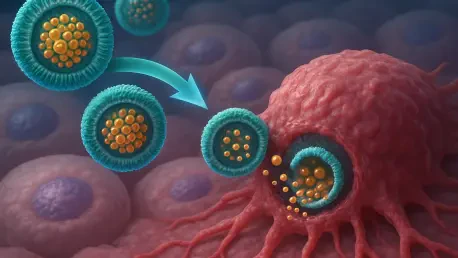
The key difference lies in our use of nanovesicles—tiny, fatty bubbles that act as a delivery vehicle. Unlike traditional formulations where the drug can scatter to unwanted areas like the liver or spleen, we’ve chemically attached paclitaxel to sphingomyelin, a natural fat found in cell membranes, to form these nanovesicles. This setup allows the drug to circulate longer in the bloodstream and accumulate more effectively at the tumor site, reducing damage to healthy tissues and minimizing side effects. It’s a game-changer in terms of precision and safety.
Paclitaxel is known for its toxicity. Can you elaborate on the challenges patients face with conventional treatments and how Paclitaxome addresses these issues?
Certainly. With conventional paclitaxel treatments, patients often experience severe side effects like nerve damage, hair loss, and low blood cell counts because the drug doesn’t discriminate between cancer cells and healthy ones. It also gets cleared from the body too quickly or ends up in organs like the liver, which can cause additional harm. Paclitaxome tackles this by using the nanovesicle structure to shield healthy tissues and direct the drug specifically to tumors. This targeted approach not only reduces toxicity but also boosts the drug’s effectiveness at the cancer site, offering a much better experience for patients.
Let’s dive into the science of nanovesicles. Can you walk us through how they work as a delivery system for paclitaxel?
Sure, I’d be happy to. Nanovesicles are essentially microscopic bubbles made of lipids, similar to the membranes of our cells. Their small size and structure make them ideal for carrying drugs through the bloodstream without being quickly cleared out. By linking paclitaxel to sphingomyelin, we create a stable nanovesicle that protects the drug and helps it penetrate tumor tissues more effectively. Tumors often have leaky blood vessels, and these nanovesicles can slip through those gaps, accumulating in the cancer site while largely bypassing healthy areas. It’s like giving the drug a GPS to find its target.
Your research showed impressive results with Paclitaxome in preclinical studies. Can you share some highlights of how it performed compared to other forms of paclitaxel?
We tested Paclitaxome against triple-negative breast cancer and advanced pancreatic cancer in mouse models, and the results were very promising. Compared to Taxol and Abraxane, Paclitaxome significantly reduced tumor growth and extended survival rates. We also engineered an enhanced version with additional modifications that further improved these outcomes. What stood out was how much better the drug stayed at the tumor site, leading to more potent effects with less overall toxicity. It’s encouraging data that suggests we’re on the right track for clinical impact.
You’ve also explored combining paclitaxel with other drugs using this nanovesicle platform. How does that enhance treatment possibilities?
Combining therapies is often key in cancer treatment, and our nanovesicle system makes this more effective. We’ve paired paclitaxel with drugs like gemcitabine and carboplatin by loading them into or onto the nanovesicle structure. For instance, we placed gemcitabine inside the core of the nanovesicle while paclitaxel was part of the outer shell. This setup allows for precise delivery of both drugs to the tumor at optimal ratios, outperforming traditional co-administration methods. In tests with triple-negative breast cancer, these combinations not only shrank tumors but also prevented recurrence and spread, which is a huge step forward.
This platform seems incredibly versatile. How do you envision it being applied to other drugs or even other diseases beyond cancer?
That’s one of the most exciting aspects of this technology. The nanovesicle approach isn’t limited to paclitaxel or even chemotherapy. We’ve already applied it to another drug, camptothecin, with success in a colon cancer model, showing that the platform can work across different drug types. Beyond cancer, we see potential in using this system for other conditions where targeted delivery is crucial, like autoimmune diseases or infections. We’re also exploring how it could pair chemotherapy with immunotherapies to boost the body’s natural defenses against cancer. The possibilities are vast, and we’re just scratching the surface.
Looking ahead, what is your forecast for the future of drug delivery systems like Paclitaxome in transforming cancer treatment?
I’m very optimistic about where this is heading. Drug delivery systems like Paclitaxome represent a shift toward personalized, precise medicine in cancer care. Over the next decade, I believe we’ll see more therapies that not only target tumors with pinpoint accuracy but also integrate multiple treatment modalities—chemotherapy, immunotherapy, and beyond—into a single platform. Our goal is to move Paclitaxome into human clinical trials and gather the data needed to make it a standard option for patients. If we can continue to refine these technologies, I think we’ll dramatically improve survival rates and quality of life for cancer patients, turning what was once a blunt tool into a finely tuned instrument.