
With the recent FDA approval of Novo Nordisk's oral version of Wegovy, the landscape of weight management treatment is poised for a monumental shift. We sat down with biopharma expert Ivan Kairatov, whose extensive background in research and development offers a unique lens on this

The intricate global network that delivers life-saving medicines has proven remarkably fragile in recent years, prompting a fundamental rethinking of how and where these complex therapies are produced. In this context, Samsung Biologics' recent acquisition of a major US-based manufacturing facility

In the years following the Supreme Court’s landmark 2022 Dobbs decision that overturned federal abortion rights, a striking and counterintuitive trend has emerged across the United States. While numerous states moved swiftly to enact severe restrictions or outright bans on the procedure, the total
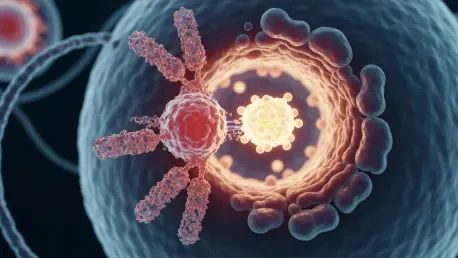
For the first time in more than a decade, the therapeutic landscape for newly diagnosed metastatic HER2-positive breast cancer has been fundamentally altered by a landmark U.S. Food and Drug Administration decision. The agency has officially approved a combination therapy featuring Enhertu and

Imagine a world where a single shot at birth could shield a child from a devastating liver disease, yet the decision to administer it hangs in a delicate balance of science, public opinion, and political will. In the United States, the hepatitis B vaccine, a cornerstone of infant health for over

Imagine a world where a persistent, hacking cough in a child could be diagnosed as whooping cough in under 20 minutes, right at the doctor’s office, preventing weeks of uncertainty and potential spread. This scenario is now closer to reality with Roche’s latest FDA-cleared point-of-care test for