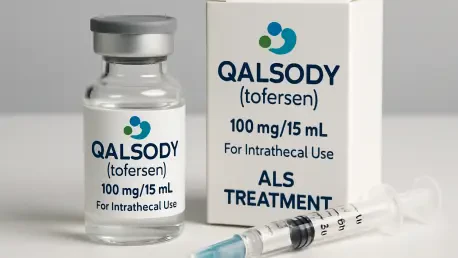
What happens when a disease as merciless as amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), strikes a tiny fraction of patients with a genetic twist that makes it even deadlier? For the roughly 2% of ALS sufferers with mutations in the SOD1 gene, life has often felt like a race against time with no finish line in sight. Enter Biogen’s Qalsody (tofersen), a groundbreaking therapy that has just secured approval from the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA). This isn’t just another drug—it’s a lifeline for a community long starved of targeted solutions, offering a flicker of possibility in the face of a devastating condition.
The significance of this milestone cannot be overstated. Affecting around 168,000 people worldwide, ALS robs individuals of muscle control, mobility, and eventually the ability to breathe. For those with SOD1 mutations, the progression is often swifter and more brutal, with no prior treatments addressing the genetic root of their plight. Qalsody’s approval marks the first time a therapy has directly tackled this specific subtype in the UK, signaling a shift toward precision medicine and raising critical questions about access and impact for rare disease communities.
A Rare Struggle: Understanding SOD1-ALS and Its Toll
ALS is a neurodegenerative juggernaut, systematically dismantling the body’s motor functions. While most cases arise sporadically with no clear cause, a small subset—about 2%—stems from mutations in the SOD1 gene, which encodes a protein meant to protect cells from damage. When faulty, this protein turns toxic, accelerating nerve cell death and hastening the disease’s grim timeline. Patients with this genetic variant often face a faster decline, watching helplessly as basic abilities slip away.
For years, the lack of targeted therapies left these individuals and their families in a void of despair. Standard ALS treatments, where they exist, focus on symptom management rather than halting the underlying destruction. The arrival of Qalsody changes the narrative, offering a direct attack on the genetic flaw driving SOD1-ALS and spotlighting the urgent need for tailored approaches in rare disease care.
The Science Behind the Solution: How Qalsody Works
At its core, Qalsody is a marvel of modern biotechnology. Classified as an antisense oligonucleotide, it zeroes in on SOD1 mRNA, the genetic blueprint for the defective protein, and disrupts its production. By curbing the toxic buildup, the drug aims to slow the relentless march of neurodegeneration—a feat previously unimaginable for this patient group. Administered through lumbar puncture by trained professionals at regular intervals, it demands precision and expertise.
Clinical evidence backs this innovation with hard data. The late-stage VALOR study revealed a significant drop in plasma neurofilament light chain—a key indicator of nerve damage—in patients treated with Qalsody compared to those on placebo. This measurable impact on disease markers suggests a real potential to alter the course of SOD1-ALS, providing a foundation for the MHRA’s approval through the International Recognition Procedure, which builds on prior authorizations in other regions since 2025.
The journey from lab to patient bedside reflects years of rigorous research and international collaboration. Regulatory nods in multiple jurisdictions underscore a global consensus on the drug’s promise, positioning it as a cornerstone in the fight against genetic ALS variants. For scientists and clinicians, this achievement is a testament to the power of targeted therapies in addressing once-untreatable conditions.
Voices from the Frontlines: Reactions to a Historic Approval
The ripple effects of Qalsody’s approval have stirred deep emotions within the ALS community. Kylie Bromley, general manager of Biogen UK and Ireland, described the moment as a “scientific triumph and a beacon of hope,” while urging reimbursement bodies like the National Institute for Health and Care Excellence (NICE), Scottish Medicines Consortium (SMC), and NHS England to adopt flexible policies. Her words highlight a dual victory—medical and moral—while acknowledging the hurdles still ahead in ensuring access.
Advocacy groups have been equally vocal in their support. UK-based charities such as MND Scotland, MND Association, and My Name’5 Doddie Foundation issued a joint statement calling the approval a “genuine breakthrough.” Their collective enthusiasm reflects years of tireless campaigning for better outcomes, amplifying the human stakes behind the regulatory win and emphasizing the profound difference this therapy could make for families grappling with SOD1-ALS.
These reactions paint a picture of unity and determination. From industry leaders to patient advocates, there’s a shared recognition that while the approval is a monumental step, the battle for widespread impact continues. Their voices serve as a reminder that behind every clinical trial and regulatory decision are real lives hanging in the balance.
Bridging the Gap: Challenges in Access and Implementation
Despite the celebration, the road to delivering Qalsody to patients is fraught with obstacles. The therapy’s administration via lumbar puncture requires specialized training and infrastructure, potentially limiting its availability to certain medical centers. This logistical challenge raises concerns about equitable access, particularly for patients in remote or underserved areas of the UK who may struggle to reach qualified facilities.
Cost and reimbursement present another hurdle. Even with MHRA approval, the therapy’s integration into public health systems hinges on negotiations with NICE, SMC, and NHS England. Advocacy groups stress that without streamlined funding mechanisms, many eligible patients could be priced out of this life-altering treatment. The call for flexibility in these processes underscores a broader tension between innovation and affordability in rare disease care.
Education also plays a pivotal role. Raising awareness about SOD1-ALS and the importance of genetic testing is essential to identify those who could benefit from Qalsody. Many patients remain undiagnosed or unaware of their genetic status, missing out on potential interventions. Bridging this knowledge gap through outreach and collaboration with healthcare providers could transform the therapy’s reach and effectiveness.
A New Chapter: Reflecting on a Milestone Moment
Looking back, the approval of Qalsody by the MHRA stood as a defining moment for the SOD1-ALS community, etching a line between despair and possibility. It marked the culmination of years of scientific grit and patient advocacy, delivering a therapy that directly confronted the genetic underpinnings of a brutal disease. The echoes of hope from Biogen leaders and charities alike resonated deeply, capturing the weight of this achievement for countless families.
Yet, the story didn’t end with approval. The path forward demanded action—pushing for genetic testing to identify eligible patients, equipping healthcare systems for complex administration, and securing funding to ensure no one was left behind. Collaborative efforts between policymakers, providers, and advocates became the next frontier, promising to turn a regulatory triumph into lasting change for those who needed it most.