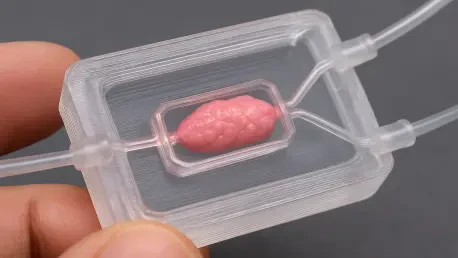
Researchers have developed a groundbreaking microfluidic chip using a single-step 3D printing process that successfully mimics the three-dimensional environments where cells grow in the human body, a development poised to revolutionize how diseases are studied and new drugs are tested. This new platform overcomes major limitations in cell culture by integrating the precise control of digital microfluidics with the biological necessity of a 3D structure. The simplified and cost-effective fabrication method makes this powerful tool more accessible for biological research, offering a more accurate way to study cells outside the body. For decades, the gold standard for cell culture involved growing cells on flat, two-dimensional plastic dishes, a method that fundamentally fails to replicate the complex, interactive environments found within living organisms. This discrepancy has long been a bottleneck in biomedical research, often leading to misleading results and failed clinical trials. The new integrated digital microfluidic platform directly addresses this challenge, offering a more physiologically relevant model that could significantly improve the predictive power of laboratory experiments.
The Challenge and the Breakthrough
Bridging the Gap in Cell Culture
The long-standing discrepancy between how cells exist in the body and how they are studied in a laboratory has presented a formidable obstacle to scientific progress. Within living organisms, cells are organized into intricate three-dimensional architectures, a structural reality that profoundly influences their behavior, communication, and overall function. In stark contrast, standard laboratory practices have historically relied on cultivating cells on flat, two-dimensional surfaces. This method fundamentally alters cellular biology, resulting in experimental outcomes that are often poor predictors of how cells will respond within actual tissues. While previous microfluidic technologies provided better control over cell culture conditions, they frequently depended on continuous fluid flow, which necessitated the use of cumbersome external pumps and involved complex, multi-step fabrication processes that limited their widespread adoption. This created a clear and pressing need for a more streamlined and integrated platform that could seamlessly combine precision with a physiologically relevant environment.
Furthermore, a specific subfield known as digital microfluidics, which excels at manipulating minute droplets with unparalleled precision, has historically been unable to support true 3D cell cultures. The primary limitation was the lack of integrated, on-chip microstructures that could house and organize cells into three-dimensional arrangements. This technical gap meant that researchers had to choose between the precise liquid handling of digital microfluidics and the biological relevance of 3D culture, but could not have both in a single, cohesive system. Fabricating such devices traditionally required multi-step lithography performed in specialized cleanroom facilities, a process that is not only time-consuming and expensive but also poses significant design constraints. These challenges collectively highlighted the urgent requirement for an innovative approach that could unify these disparate functionalities into a single, accessible, and powerful tool for modern biological research, setting the stage for a significant technological leap forward.
A Unified Fabrication Process
The innovative solution presented by researchers is an integrated digital microfluidic chip featuring 3D-printed microstructures fabricated directly onto the device’s electrodes. The core breakthrough lies in a manufacturing strategy that merges digital microfluidics and 3D microstructures into a single, cohesive device through a streamlined, one-step process. Instead of relying on traditional, resource-intensive methods, the research team employed an advanced 3D printing technique known as projection stereolithography. This method allowed them to fabricate all the essential components—including the dielectric layer, confinement fences, and micro-well arrays—in a single, unified operation. This approach dramatically simplifies the chip’s production, significantly reducing both the complexity and the cost associated with its creation. By eliminating the need for specialized cleanroom facilities and intricate fabrication protocols, this technique democratizes access to advanced cell culture technology, making it available to a much broader scientific community.
This simplified manufacturing workflow not only makes the technology more accessible but also provides for precise and highly customizable control over the three-dimensional cellular microenvironment. The one-step 3D printing process allows for rapid prototyping and easy modification of the chip’s design, enabling researchers to tailor the microstructures to specific experimental needs. For instance, the size, shape, and arrangement of the micro-wells can be easily adjusted to study different cell types or to model various tissue architectures. This level of customization is difficult and costly to achieve with conventional fabrication methods. The ability to create sophisticated, bespoke microenvironments on a chip represents a significant advancement, providing a powerful platform for investigating complex biological processes with greater accuracy and control. The balance between functional sophistication and manufacturing simplicity is a key advantage that could accelerate the adoption of 3D cell culture technologies in laboratories worldwide.
Inside the Chip Function and Findings
From Droplets to Tissues
The resulting chip demonstrates a wide range of advanced capabilities, validating its potential as a transformative tool for cell biology. The research team successfully optimized key operational parameters, including the applied voltage, the geometry of the electrodes, and the height of the microstructures, to ensure consistent and reliable droplet actuation. As a result, the chip can expertly perform all essential digital microfluidic operations, such as transporting, splitting, and merging droplets with high precision. Critically, these functions are not limited to the device’s flat surfaces; the chip can reliably manipulate droplets across its 3D-printed topographies as well. This precision allows researchers to guide droplets containing cell suspensions into the designated micro-wells with remarkable accuracy and efficiency. This controlled delivery is essential for ensuring that cells are deposited correctly within the 3D structures, which is the first step toward creating organized, tissue-like formations for subsequent study.
Once the cells are successfully captured and confined within these 3D microstructures, they rapidly self-assemble into compact, three-dimensional spheroids. This process of self-assembly is a key indicator of a more physiologically relevant environment, as it mimics the natural tendency of cells to form structured communities within the body. The chip’s design facilitates this process by providing a scaffold that encourages close cell-to-cell contact and communication, which are crucial for the development of functional tissue-like structures. The ability to controllably and repeatedly generate these cell spheroids on a single, integrated platform is a major step forward from conventional methods, which are often less reliable and offer limited control over the final cellular architecture. The platform effectively bridges the gap between precise liquid handling and the creation of biologically meaningful 3D cell models, all within a miniaturized and automated system.
A New Frontier for Biomedical Research
Experimental results have unequivocally confirmed the platform’s efficacy and its biological superiority over conventional methods. The 3D cell spheroids formed on the chip exhibited significantly enhanced cell-to-cell interactions and a more organized, tissue-like structure when compared to their counterparts grown in traditional 2D cultures. Comprehensive viability and proliferation assays demonstrated that the cells remained healthy and continued to grow over extended periods, with stable operation and high cell viability observed for up to 72 hours. Furthermore, detailed imaging analyses revealed the formation of dense, multicellular architectures that closely mimic the intricate structure of in-vivo tissues. This finding underscores the platform’s remarkable ability to create more biologically relevant models, which are essential for generating data that can be more accurately translated to human health. The consistency and reproducibility of these results highlight the robustness of the integrated chip as a reliable research tool.
The development of this platform represented a multifaceted solution to a major bottleneck that had long hindered progress in microfluidic cell culture. By integrating 3D microstructures directly into a digital microfluidic chip through a simplified fabrication workflow, the researchers created a uniquely powerful tool that combined precise droplet control with a physiologically relevant environment. This technology was poised to have immediate implications across several critical areas of biomedical research. In drug screening, it promised more accurate predictions of a compound’s efficacy and toxicity. For fields like cancer biology and tissue engineering, it offered a means to study complex multicellular structures in a controlled manner. Future plans aimed to enhance the platform by reducing operating voltages, integrating real-time sensing capabilities, and enabling the co-culture of multiple cell types, which would pave the way for creating even more complex tissue models and further narrowing the gap between laboratory experiments and living biological systems.