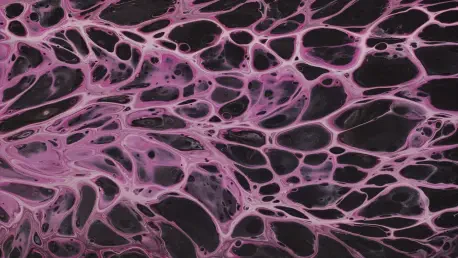
In recent years, the field of tissue engineering has witnessed groundbreaking advancements, one of which is the development of 3D-printed collagen scaffolds by researchers at the University of Pittsburgh. This innovative technology, named CHIPS—collagen-based, high-resolution, internally perfusable structures—aims to replicate natural cellular environments, thereby enabling cells to naturally grow, interact, and form functional tissues. This marks a significant advancement over traditional silicone-based models that have been used for such purposes. By endeavoring to simulate the complexities of the human body’s internal infrastructure, this advancement is poised to elevate the capabilities of in vitro disease modeling and tissue engineering, potentially transforming current medical practices and therapeutic approaches.
Revolutionary Concept in Tissue Simulation
The cornerstone of this innovative research is the understanding that cells, when placed within an appropriately designed environment, possess an intrinsic capacity to organize and function similarly to their natural counterparts. Key to this is the creation of scaffolds that closely mimic the intricate structures found in the body. The CHIPS scaffold, developed through the collaboration of Assistant Professor Daniel Shiwarski and his team at the University of Pittsburgh with Adam Feinberg from Carnegie Mellon University, serves this purpose by being a high-resolution, internally perfusable construct fashioned entirely of collagen. This work, detailed in the publication Science Advances, reflects a considerable shift toward utilizing biological materials in creating sophisticated tissue models. This methodology allows researchers to study cellular behavior in vitro with a precision previously unattainable using synthetic platforms, paving the way for more nuanced insights into cellular processes and their implications for human health.
Overcoming Limitations of Traditional Models
The shift from traditional silicone-based to collagen-based materials marks a considerable stride in addressing the limitations inherent in synthetic systems. Traditional microfluidic devices have served as beneficial tools in simulating human disease conditions but have been constrained by their inability to accurately reflect the biological intricacies of natural tissues. Synthetic materials often lack the necessary biological cues that cells require to thrive and function optimally. By employing natural biomaterials like collagen, the CHIPS platform has succeeded in more effectively supporting cellular growth and organization, vital for crafting accurate disease models. This approach holds particular promise for advancing the understanding and treatment of conditions such as diabetes and hypertension, where the functionality of replacement tissues is essential. Harnessing additive manufacturing and tissue engineering principles, this research seeks not only to replace and repair biological tissues but also to advance disease modeling, thereby overcoming the limitations faced by traditional methods.
Breakthrough in Living Tissue Models
The innovation embodied in CHIPS allows for the creation of living tissue models that mimic the complexity and functionality of actual human organs. By embedding living cells within a collagen matrix, researchers have developed models where cells can grow, interact, and organize into structured tissues. An illustrative example of this capability is the development of pancreatic tissues that not only sense glucose levels but also secrete insulin in response, mimicking the natural physiological processes found in the human body. This capability highlights the tremendous potential for treating diseases such as type 1 diabetes, offering new avenues for regenerative medicine that could ultimately enable the manufacturing of human organs. These models stand as a transformative tool for both understanding the underlying biology of diseases and devising new therapeutic strategies. This breakthrough underscores the importance of creating environments that nurture cellular growth and functionality, which are pivotal in moving beyond the confines of traditional disease treatment and toward innovative regenerative solutions.
Achieving Realistic Vascular Networks
An integral part of this pioneering research is the creation of three-dimensional vascular networks within a soft, organic framework, setting a groundbreaking precedent over traditional models restricted to flat, two-dimensional constructs. The ability to develop intricate networks that mirror those found in nature, such as helical vascular formations inspired by DNA structures, represents a significant leap forward. These networks incorporate the benefits of microfluidic technology, such as fluid flow control, with the inherent properties of natural biomaterials and the cells’ own predisposition for growth. The synthesis of these elements enables the crafting of a more authentic and functional vascular system that supports detailed study of vascular diseases and offers potentially better therapeutic strategies. This capability promises to transform not only disease modeling but also the broader context of tissue engineering, providing insights that could lead to novel treatments for cardiovascular issues. By advancing beyond 2D limitations to achieve more lifelike 3D networks, this research moves closer to creating comprehensive models of the human vascular system.
Open Science and Future Prospects
The commitment to open science in this research project enhances possibilities for innovation and collaboration across the scientific community. By making all models and designs freely accessible, researchers encourage widespread adoption and adaptation of these advanced technologies, paving the way for further developments in crafting human-based disease models. This approach not only emphasizes the importance of collaborative efforts in advancing scientific knowledge but also positions these models as potential alternatives to animal testing. More accurate and human-relevant data can be gathered using these models, offering better insights into how diseases function and progress, particularly for complex vascular conditions. As science continues to evolve, the sharing of resources and methodologies becomes crucial in addressing global health challenges. This open-access policy nurtures an inclusive environment where research can build upon existing findings, fostering a landscape where continuous scientific advancement becomes a shared endeavor among global researchers, aimed at enhancing human health outcomes through cutting-edge technology.
Bridging Research Gaps
The foundation of this pioneering research hinges on the concept that cells, when situated in a precisely designed environment, can naturally organize and function akin to their natural state. A critical aspect of this lies in developing scaffolds that closely mimic the body’s intricate structures. The CHIPS scaffold, created by Assistant Professor Daniel Shiwarski and his team at the University of Pittsburgh with Adam Feinberg from Carnegie Mellon University, epitomizes this approach. It is a high-resolution, internally perfusable structure made entirely of collagen. This collaboration, discussed in the journal Science Advances, marks a significant shift toward using biological materials to craft advanced tissue models. Such a technique enables researchers to examine cellular behavior in vitro with unprecedented precision, a feat not possible with traditional synthetic platforms. This advancement holds promise for providing deeper insights into cellular processes, paving the way for breakthroughs in understanding human health and disease mechanisms.