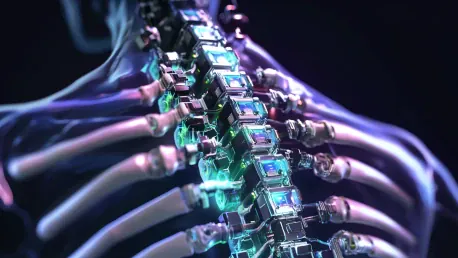
In the laboratories of Matricelf, located in Nes Ziona, Israel, an innovative development endeavor is underway, holding the potential to revolutionize the treatment of spinal cord injuries. Established in 2019 by Professor Tal Dvir and Dr. Alon Sinai, Matricelf is at the forefront of creating autologous neural implants. These technologies aim to replace damaged spinal cord areas, offering the possibility of functional recovery for individuals with paralysis. This monumental leap in medical science could change the lives of millions globally, a sentiment underscored by Matricelf CEO Gil Hakim. Hakim articulates the company’s mission as restoring sensations and bodily function control, with the ultimate aspiration of enabling paralyzed individuals to regain their ability to walk independently. The convergence of science, medicine, and humanitarian efforts signifies a fundamental shift in medical treatment approaches for spinal cord injuries.
Innovative Technology for Functional Recovery
The Science Behind Autologous Implants
The crux of Matricelf’s innovation lies in the development and application of an implant derived from the patient’s own body. Fat tissue and blood samples from the patient are utilized to engineer functional nerve tissue through advanced tissue engineering techniques. This autologous approach prevents immune rejection and increases the likelihood of successful recovery. The newly created nerve tissue is implanted at the site of injury, effectively forming a biological bridge that reinstates nerve communication, subsequently restoring movement and sensation.
In this technological process, the extracted fat tissue and blood samples are first transformed into induced pluripotent stem cells (iPSCs). These stem cells are then differentiated into neural progenitor cells that can develop into the functional nerve tissue. This precise engineering of nerve tissues mimics the natural growth and repair mechanisms of the human body. Such a tailored approach ensures the compatibility of the implant, as it is inherently derived from the same body it intends to heal. The seamless integration of these implants promotes efficient and enduring recovery, offering a new lease on life to individuals grappling with spinal cord injuries.
Advantages of Autologous Implants
Using the patient’s own cells to create the implant offers several advantages. Firstly, it eliminates the risk of immune rejection, a common issue with donor tissues. Secondly, the process ensures a perfect match in terms of tissue compatibility, enhancing the chances of successful integration and function. Lastly, this method leverages the body’s natural healing processes, potentially leading to more effective and lasting recovery.
The autologous technique also provides a significant psychological benefit for patients, as they feel reassured knowing that their treatment comes from their own body. This familiarity can reduce anxiety associated with foreign body implants and improve the overall acceptance of the therapy. Moreover, the application of personalized medicine principles ensures that the treatment is unique to each individual’s physiological condition, paving the way for more targeted and precise medical interventions. The advances in tissue engineering embodied by these autologous implants represent a significant leap forward in the field of regenerative medicine, promising a future where spinal cord injuries are no longer a life sentence.
Drawing Confidence from Experimental Success and Economic Necessity
Promising Initial Results
Despite being in the development phase, Matricelf went public on the Tel Aviv Stock Exchange in 2021, propelled by promising initial results and substantial investor confidence. The basis of this confidence is rooted in a successful pilot experiment conducted on a rat model with a compression injury, a type that commonly occurs in human car accidents. In this experiment, the implantation of a human neural implant resulted in the rat regaining its ability to walk and even run. This experimental success mirrors the biological mechanisms underlying human paralysis caused by interrupted signal transmission between the brain and muscles.
Such experimental triumphs are not just limited to rats. These findings have significant implications for human applications, demonstrating that engineered neural implants can recreate the intricate network of nerves required for movement. The scalability of this technique to larger and more complex organisms like humans is a promising step toward clinical trials. The rat study, which was meticulously documented and peer-reviewed, serves as a robust foundation upon which further research and human applications are being built. It has garnered attention and optimism from the scientific community and financial investors alike, signifying a groundbreaking moment in spinal cord injury treatment.
Economic Implications
From an economic standpoint, the financial burden of treating paralyzed individuals is staggering, with annual costs in the United States estimated at approximately $50 billion. Each patient’s treatment can amount to millions of dollars, underscoring an urgent need for innovative solutions like Matricelf’s. The combination of significant market demand and the compelling medical potential of this technology adds to its feasibility and appeal.
The economic implications extend beyond immediate healthcare costs. Effective treatment options can significantly reduce long-term care expenses and enable individuals to contribute to the workforce, thereby enhancing economic productivity. Additionally, with a potential reduction in dependency on pain management and ancillary therapies, the overall quality of life for patients could see substantial improvement. Insurance companies and public health systems are likely to support such cutting-edge treatments as they promise reduced long-term expenditures and better patient outcomes. Thus, Matricelf’s innovations could redefine the economic landscape of spinal cord injury treatments, offering both medical and financial relief.
Expertise in Tissue Engineering: Leadership and Innovation
Pioneering Minds Behind Matricelf
Prof. Tal Dvir, Matricelf’s co-founder and chief scientist, is internationally recognized for his contributions to biotechnology, tissue engineering, and regenerative biomedicine. Leading prestigious research centers at Tel Aviv University, Prof. Dvir’s pioneering work in creating autologous implants from the patient’s cells led to Matricelf’s inception. Dr. Alon Sinai, a noteworthy biotech entrepreneur, joins him in this endeavor.
With a distinguished career marked by numerous accolades and breakthroughs, Prof. Dvir’s vision transcends conventional medical paradigms, steering the company towards paths previously deemed achievable only in theoretical models. His leadership and expertise are instrumental in driving Matricelf’s research, ensuring that the technology not only meets but exceeds the rigorous standards of global scientific communities. Dr. Alon Sinai complements Dvir’s scientific prowess with his entrepreneurial acumen, bringing a wealth of industry experience to propel Matricelf’s innovations from laboratory to market. Together, their dynamic collaboration symbolizes a rare blend of visionary science and strategic execution.
Visionary Leadership
CEO Gil Hakim describes this cutting-edge technology as reminiscent of science fiction, emphasizing his strong belief in its transformative potential. Matricelf’s team, composed of field-leading experts, fuels his optimism. Hakim sees their work as a ground-breaking new treatment approach aimed at offering hope to individuals who have suffered severe, life-altering spinal cord injuries.
Hakim’s visionary leadership is reflected in his commitment to fostering a culture of innovation and excellence within Matricelf. He actively engages with the broader scientific community, healthcare providers, and regulatory bodies to build a robust support system for the company’s groundbreaking work. Under his stewardship, Matricelf not only focuses on technical advancements but also prioritizes patient care, ethical considerations, and financial viability. His unwavering dedication to pioneering new frontiers in medical science ensures that Matricelf remains at the forefront of therapeutic innovations, poised to make significant strides in treating spinal cord injuries and beyond.
Potential Beyond Spinal Cord Injuries
Expanding Treatment Capabilities
The platform developed by Matricelf is not limited to spinal cord injuries alone. The same tissue engineering techniques can be adapted to create various types of nerve tissues, potentially offering treatments for other neurological damages such as head injuries and strokes. To expand their treatment capabilities, Matricelf has secured a unique license from Tel Aviv University for tissue printing technologies. Under this auspice, Prof. Dvir was the first to print a tissue-based heart structure in 2019 and has since advanced to printing blood vessels. This technology holds promise for a broad spectrum of regenerative treatments.
These advancements signify the versatility and adaptability of Matricelf’s proprietary technology. By integrating tissue printing techniques, the company can tailor different types of cellular structures specific to various medical conditions. This not only broadens the scope of potential treatments but also aligns with the growing trend towards personalized medicine. With Prof. Dvir at the helm of these innovative practices, Matricelf is prepared to address a wide range of neurological afflictions, leveraging their groundbreaking methods to potentially treat conditions that have long eluded effective intervention. This broad application paints a future where regenerative medicine is a cornerstone of medical practice.
Future Prospects
With preliminary approval from the Israeli Ministry of Health, Matricelf anticipates beginning treatments under compassionate care by 2025. This forthcoming milestone involves obtaining fat and blood samples from patients, transforming them into unique nerve tissues within a six-month period, and then surgically implanting these tissues at the injury site. Early results may be observable within a few months post-implantation. The outcome holds potential for profound impacts on patients’ lives, transforming paralysis paradigms.
The initiation of these human trials marks a pivotal moment in the journey of Matricelf’s visionary projects. As these trials progress, continuous monitoring and analysis will provide invaluable data to refine and optimize the treatment process further. The success in human applications could pave the way for extensive clinical use and potentially alter the standard of care for neurological damage worldwide. By setting ambitious yet attainable milestones, Matricelf underscores its commitment to driving forward the boundaries of medical science, ensuring that their innovative treatments are accessible and effective for those in dire need.
Overview
Matricelf’s groundbreaking innovation focuses on creating implants derived from a patient’s own body. This involves using fat tissue and blood samples to develop functional nerve tissue through advanced tissue engineering. By using the patient’s own cells, this autologous method avoids immune rejection and boosts the chance of a successful recovery. The engineered nerve tissue is implanted at the injury site, creating a biological bridge that restores nerve communication, thereby reinstating movement and sensation.
During this process, extracted fat tissue and blood samples are converted into induced pluripotent stem cells (iPSCs). These stem cells are then differentiated into neural progenitor cells, which grow into functional nerve tissue. This precise engineering simulates the body’s natural growth and repair mechanisms. A tailored approach ensures the implant’s compatibility because it originates from the same body it aims to heal. The seamless integration of these implants supports effective and lasting recovery, giving new hope to individuals suffering from spinal cord injuries.