

Despite advances in medical technology, early diagnosis of breast cancer remains a formidable challenge, with significant implications for patient outcomes and healthcare systems. Researchers have continually searched for innovative solutions to enhance predictive accuracy and diagnostic

Artificial Intelligence (AI) is making significant inroads into healthcare call centers, a sector historically reliant on high-volume labor in regions like the Philippines. These centers have long been crucial in supporting healthcare operations, including managing chronic illness assistance and

The burgeoning field of microbiome science holds significant promise for revolutionizing clinical care. At the core of this research is an exploration of the human microbiome, the vast community of microorganisms inhabiting our bodies. Recent technological advances have paved the way for mapping
In recent scientific developments, researchers from Kiel University and the University of Hamburg have unveiled a pioneering method to direct atomic movement using magnetism. Traditionally, the movement of individual atoms across a surface was assumed random, a belief now challenged by these
Amid growing public health challenges, the introduction of Generative Artificial Intelligence (GenAI) offers a transformative breakthrough in developing youth health campaigns aimed at issues like vaping. GenAI's efficiency has been spotlighted, showing the potential to drastically reduce the time

In the rapidly advancing field of biofabrication, a groundbreaking technique has emerged from a team of scientists at the Renaissance School of Medicine at Stony Brook University. This innovation, known as TRACE (Tunable Rapid Assembly of Collagenous Elements), signifies a major leap forward in
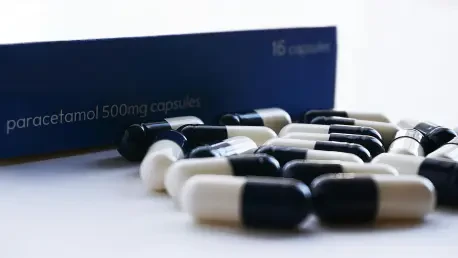
Chelonia Healthcare Limited has issued a recall for two specific batches of Paracetamol 500mg tablets upon the guidance of the Medicines and Healthcare Regulatory Authority (MHRA). This action arises from reports by healthcare professionals who identified discolored tablets in some of the packages.

A recent study conducted by the Cleveland Clinic sheds light on the real-world challenges faced by injectable medications used for obesity treatment, namely semaglutide and tirzepatide. These medications, approved by the FDA for both obesity management and type 2 diabetes care, are marketed under

In the realm of genetic engineering and de-extinction science, researchers find themselves on the cusp of extraordinary breakthroughs as they attempt to resurrect the woolly mammoth, a species that disappeared from Earth thousands of years ago. As of 2025, an unexpected byproduct of this ambitious
With a background steeped in biopharmaceutical innovation, Ivan Kairatov is a leading expert in oncology therapeutics. His insights into the development and approval of new cancer treatments are invaluable, especially regarding the European Commission's recent decision on AstraZeneca's Calquence
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy