
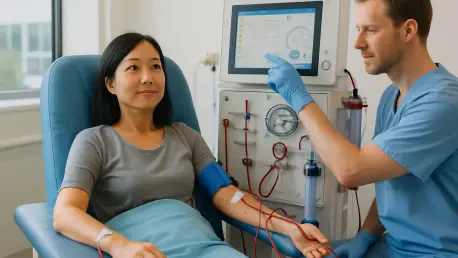
In a landscape where millions of patients with advanced chronic kidney disease (CKD) struggle with limited and often burdensome treatment options, a groundbreaking development has emerged to potentially transform their lives. Opterion Health AG, a clinical-stage biopharmaceutical company
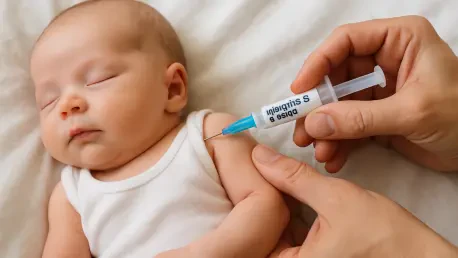
What if a single policy change could jeopardize decades of progress in safeguarding children from a silent, deadly virus, and what would be the consequences of such a shift? The debate over delaying the hepatitis B vaccine from birth to age 4 has erupted into a heated controversy, pitting public

In a groundbreaking stride toward health equity, a pair of visionary sisters has introduced an innovative solution to address the longstanding disparities in healthcare for Black women, marking a significant milestone in medical accessibility. Dr. Jacqueline Philips and Lorraine Phillips have
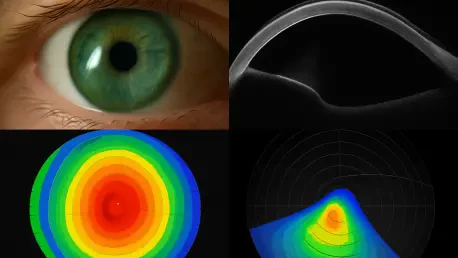
What if a simple eye scan could forecast a patient’s risk of losing vision to a stealthy, progressive condition long before irreversible damage sets in, offering a lifeline to preserve sight? This question is no longer a distant dream but a tangible reality emerging in the field of ophthalmology.
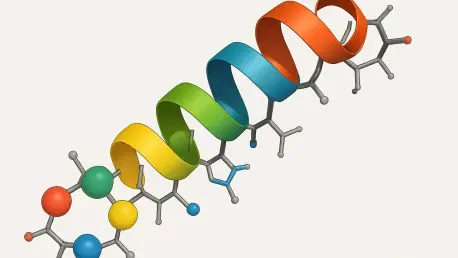
The relentless battle against cancer continues to drive scientific innovation, with researchers exploring every possible avenue to develop treatments that can effectively target cancer cells while sparing healthy tissues from harm. One particularly promising approach involves anticancer peptides

In an era where robotics and artificial intelligence (AI) are transforming industries at an unprecedented pace, staying informed about the latest breakthroughs and trends is more crucial than ever. Imagine a world where autonomous systems seamlessly integrate into daily life, from manufacturing

I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, and a deep understanding of technology and innovation in the industry. Today, we’re diving into the groundbreaking world of AI-driven drug discovery, focusing on a

In the ongoing battle against cancer, clinical trials remain a cornerstone for advancing new therapies, yet a staggering reality persists: only about 7.1% of U.S. adults diagnosed with cancer currently participate in these critical studies, hampering the pace of drug development. This alarmingly
Imagine a world where the very genetic traits often tied to challenges like autism spectrum disorder (ASD) are the same ones that helped humanity rise above other species in cognitive prowess, as suggested by a remarkable study led by Alexander Starr, a PhD student at Stanford University, and
As America's population continues to age at an unprecedented rate, with over 55 million individuals aged 65 and older, the demand for innovative aging care solutions has reached a critical juncture, posing unique challenges to healthcare systems, families, and communities grappling with gaps in
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy